Fig. 9.
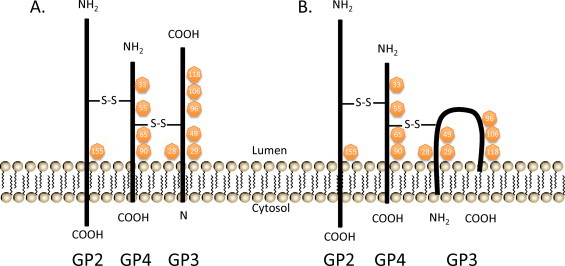
Two predicted models for the disulfide-bonded structure of the covalently linked GP2—GP3—GP4 heterotrimer. These alternative models differ in membrane topology of GP3, which is currently uncertain. The GP3 protein may be either a class II membrane protein (A) or a class IV membrane protein (B). The intermolecular cystine bridges are depicted arbitrarily with disulfide bonds (S—S) since the Cys positions have not yet been determined. N-glycosylation sites are indicated in orange circles with corresponding amino acid positions.
Modified from Wieringa et al. (2003b) with permission.
