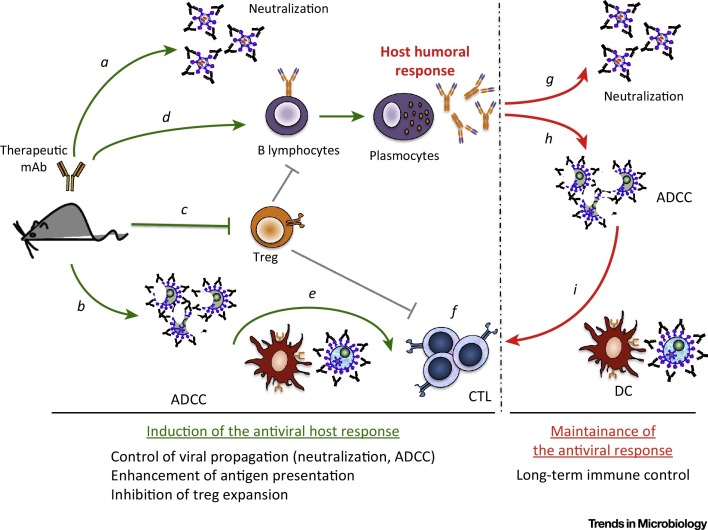
Figure 2.
Key Figure: Induction of Life-Long Protective Immunity Against FrCasE by Neutralizing Antiviral Monoclonal Antibodies (mAbs)
The administration, to FrCasE-infected mice, of a neutralizing antiviral IgG2a mAb directed to the viral Env glycoprotein limits viral propagation through neutralization of virus (a) and elimination of infected cells (b). At the same time, the immune complexes (ICs) formed with the virus, and probably more importantly with infected cells expressing Env at their surface, prevent the expansion of regulatory T cells (Treg cells) (c), which is necessary for the development of protective humoral (d) and cellular (e, f) responses by the host. In particular, the ICs formed between the therapeutic mAbs and infected cells activate dendritic cells (DCs), leading to an enhanced virus-specific CD8 T-cell response (f). Once the therapeutic antibody has been eliminated from treated mice, both arms of the adaptive immune system contribute to viral propagation control, as the endogenous antiviral antibodies, induced by the immunotherapy, control viral propagation (g, h) and contribute to the maintenance of a T-cell memory response. This requires the formation of ICs engaging residual infected cells and activation of DCs (i). Abbreviation: ADCC, antibody-dependent cell-mediated cytotoxicity.