Scheme 1.
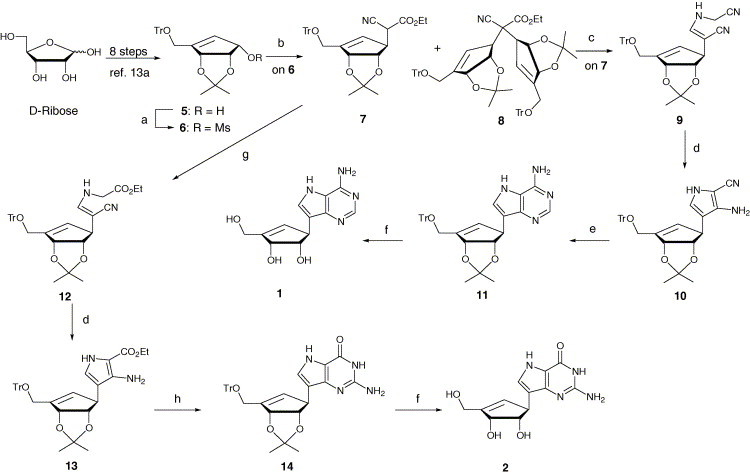
Reagents and conditions: (a) MsCl, Et3N, CH2Cl2, 0 °C, 1 h; (b) NCCH2CO2Et, NaH, THF, rt then to 55 °C, 40 h; (c) i—DIBAL-H, Et2O, −78 °C, 30 min; ii—H2NCH2CN·H2SO4, NaOAc·3H2O, MeOH, rt, 24 h; (d) i—ClCO2Et, DBU, CH2Cl2 0 °C then to reflux, 6 h; ii—K2CO3, MeOH, rt, 1 h; (e) HC( NH)NH2·AcOH, EtOH, reflux, 8 h; (f) i—12% HCl/MeOH, rt, 2 h; ii—NaHCO3/MeOH, rt, 2 h; (g) i—DIBAL-H, Et2O, −78 °C, 30 min; ii—H2NCH2CO2Et·HCl, NaOAc·3H2O, MeOH, rt, 24 h; (h) i—BzN C S, CH2Cl2, 0 °C, 1 h; ii—MeI, DBN, CH2Cl2, rt, 2 h; iii—NH3/MeOH, 95 °C, 16 h.
