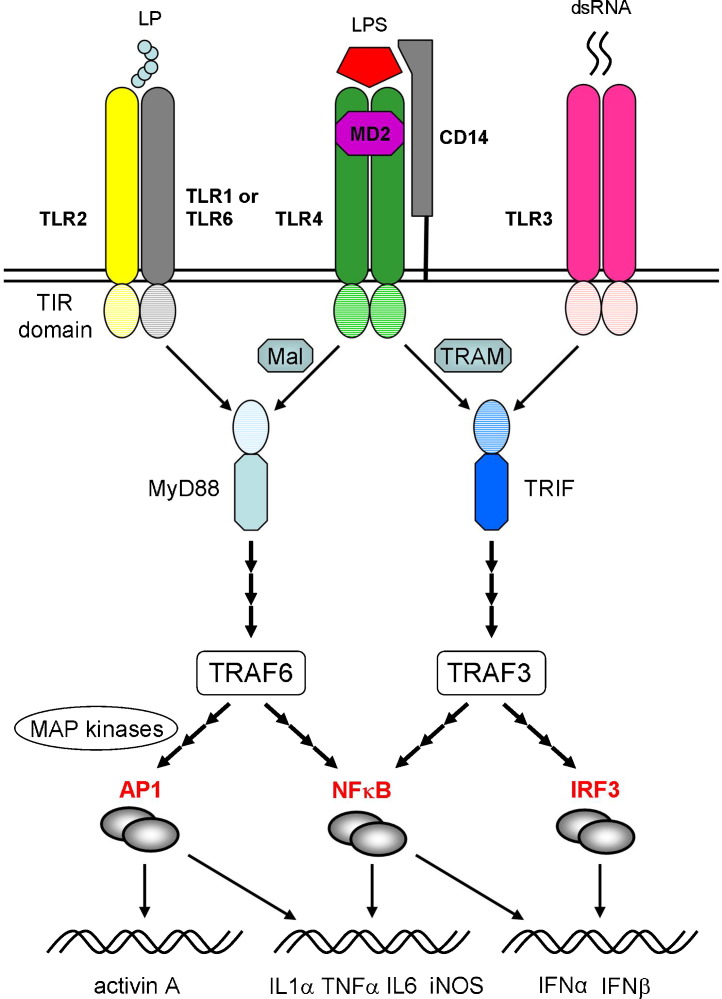
Fig. 2.
Toll-like receptors (TLRs) and signalling pathway interactions. The TLRs respond to a variety of pathogen-related molecules, usually forming homo- or heterodimers during signalling. TLR2 can self-associate or combine with either TLR1 or TLR6 to mediate responses to various bacterial lipoprotein (LP) classes. TLR4 forms a complex with the co-receptor proteins, MD2 and CD14, to facilitate binding of bacterial LPS. The TLRs are sub-divided into cell surface receptors (e.g. TLR2 and 4), which largely respond to bacterial proteins, lipoproteins and lipopolysaccharides, and intracytoplasmic endosomal receptors (e.g. TLR3), which recognise bacterial and viral nucleic acids, such as viral double-stranded RNA (dsRNA). Most TLRs signal via the adaptor protein, MyD88 (myeloid differentiation primary response protein 88), except TLR3, which acts through the adaptor protein TRIF (TIR domain-containing adaptor-inducing interferon β). Uniquely, TLR4 can interact with either MyD88 or TRIF, through engagement of the adaptor proteins, Mal (MyD88 adaptor-like) or TRAM (TRIF-related adaptor molecule). Downstream signalling involves the tumour necrosis factor receptor-associated factors 3 and 6 (TRAF3 and TRAF6), activation of the transcription factors, NFκB (nuclear factor kappaB) and IRF3 (interferon regulatory factor 3), or induction of the mitogen-activated protein kinases (MAP kinases) Jnk (Jun N-terminal kinase) and p38 and production of the Fos/Jun transcription factor AP1 (activated protein 1). These transcription factors interact to induce the expression of multiple pro-inflammatory genes, including interleukin (IL) 1α, tumour necrosis factor α (TNFα), inducible nitric oxide (iNOS), or the type 1 interferons (IFNα, IFNβ). Note that many details of the signalling pathways have been omitted or truncated for the sake of simplicity.