Graphical abstract
Keywords: Inflammation, Cyclooxygenase-2, Houttuynia cordata, NS398
Abstract
Ethnopharmacological relevance
Houttuynia cordata Thunb. (Saururaceae; HC) has been long used in traditional oriental medicine for the treatment of inflammation diseases. Modern research has implicated inducible cyclooxygenase-2 (COX-2) as a key regulator of the inflammatory process.
Aim of the study
In the present study, we aimed to investigate the effect of HC on COX-2. We examined the effects of HC on lipopolysaccharide (LPS)-induced prostaglandin (PG) E2 production, an indirect indicator of COX-2 activity, and COX-2 gene and protein expression in mouse peritoneal macrophages.
Materials and methods
LPS-induced mouse peritoneal macrophages were employed as an in vitro model system. LPS-induced PGE2 production was assessed by enzyme-linked immunosorbant assay and COX-2 protein expression was assessed by Western blot assay.
Results
The results showed that HC was able to inhibit the release of LPS-induced PGE2 from mouse peritoneal macrophages (IC50 value: 44.8 μg/mL). Moreover, the inhibitory activity of HC essential oil elicited a dose-dependent inhibition of COX-2 enzyme activity (IC50 value: 30.9 μg/mL). HC was also found to cause reduction in LPS-induced COX-2 mRNA and protein expression, but did not affect COX-1 expression. The non-steroidal anti-inflammatory drug (NSAID) and specific COX-2 inhibitor NS398 functioned similarly in LPS-induced mouse peritoneal macrophages.
Conclusion
Taken together, our data suggest HC mediates inhibition of COX-2 enzyme activity and can affect related gene and protein expression. HC works by a mechanism of action similar to that of NSAIDs. These results add a novel aspect to the biological profile of HC.
1. Introduction
Inflammation is a natural host-defensive process in the innate immune response. Nonsteroidal anti-inflammatory drugs (NSAIDs) have proven to be significantly efficacious in the treatment of various disorders with an inflammatory component. NSAIDs function by binding to the cyclooxygenase (COX) enzymes to inhibit the production of prostaglandins from the substrate arachidonic acid. The prostaglandins that are otherwise normally produced by COXs have been implicated in a wide array of physiological events, including progression of inflammation, immunomodulation, and transmission of pain signals (Vane and Botting, 1998). COX-1 is known to be constitutively expressed in mouse peritoneal macrophages and RAW264.7 cells, while COX-2 is overtly induced upon stimulation by lipopolysaccharide (LPS) and pre-inflammatory cytokines, such as interferon-gamma (Mitchell et al., 1994, Guastadisegni et al., 2002, Simmons et al., 2004). Induced inhibition of COX-1 in humans and animal models has resulted in undesirable side-effects, whereas COX-2 inhibition has generated therapeutic effects in management of pain, inflammation, cancer and neuropathologic conditions, such as Alzheimer's and Parkinson's disease (Loren, 2002, Jachak, 2006, Blobaum and Marnett, 2007).
In light of the above findings, COX-2 has become the focal point for the development of anti-inflammatory and anticancer drugs. NSAIDs, non-selective non-aspirin NSAIDs and COX-2 selective inhibitors are currently used to treat a wide array of inflammatory disorders and for cancer prevention (Shan et al., 2004, Jack et al., 2009, Thun and Blackard, 2009). Selective inhibitors of COX-2, however, have been associated with a small but definite risk of myocardial infarction and stroke. The associated gastric damage of conventional NSAIDs and the recent withdrawal of selective COX-2 inhibitors from the market due to their adverse cardiovascular side-effects have generated a considerable impetus to develop alternative anti-inflammatory agents (Debabrata, 2002, Ortiz, 2004, Coruzzi et al., 2007). Plant-derived natural agents may be useful in this regard.
Houttuynia cordata Thunb. (Saururaceae; HC), is a time-honored traditional Chinese medicine (TCM). HC has been associated with a broad range of pharmacological activities, including antiviral (Hayashi et al., 1995, Chiang et al., 2003), antileukemic (Chang et al., 2001, Chiang et al., 2004, Kwon et al., 2003), antioxidative (Nuengchamnong et al., 2009), antianaphylaxis and anticancer effects (Han et al., 2009, Lin et al., 2009, Li et al., 2005, Tang et al., 2009). Recently, several scientific studies have provided data to support and explain the particular anti-inflammatory activities of HC (Park et al., 2005, Lu et al., 2006a, Lu et al., 2006b, Ji et al., 2009).
HC was demonstrated to be one of eight types of TCM that play a unique role in facilitating the resolution of severe acute respiratory syndrome (commonly known as SARS), owing to its abilities to diminish inflammation (Lau et al., 2008, Zhang and Chen, 2008). Medicinal properties that have been attributed to the drug are believed to be harbored by its volatile oil component (Hayashi et al., 1995, Lu et al., 2006a). Some studies have attempted to characterize the essential oil composition of Houttuynia cordata, extracting the essential oils by steam distillation and performing gas-chromatography–mass-spectrometry (Xu et al., 2005, Lu et al., 2006a, Chen et al., 2007).
Although it has been shown that HC has anti-inflammatory activity, the precise molecular mechanism underlying this effect is not well understood. In the study presented herein, we addressed the possibility of whether HC acts by interfering with the actions of COX-2. The cell model of primary LPS-induced murine peritoneal macrophages was employed since these cells are known to express high levels of COX-2. We determined the effects of HC essential oil on COX-2 mRNA and protein expressions and described the possible mechanism underlying the anti-inflammatory activity of HC.
2. Materials and methods
2.1. Materials and reagents
Houttuynia cordata Thunb. was purchased from the TCM Store (Xi’an, PR China). Verification of identity was carried out by Professor Yingli Li (School of Medicine, Xi’an Jiaotong University, Xi’an, China). A voucher specimen (No. HC2008005) was deposited in the herbarium of the School of Medicine, Xi’an Jiaotong University, Xi’an, China. RPMI 1640 medium was purchased from Gibco (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO), 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), LPS, thioglycollate broth, acetylsalicylic acid, arachidonic acid, trypan blue dye and trypsin were purchased from Sigma–Aldrich Biotechnology (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Lanzhou-min sea Biological Engineering Co, Ltd. (Lanzhou, China). Sodium thioglycollate was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Anti-phosphol-COX-1 antibody, anti-phosphol-COX-2 antibody and anti-β-actin antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Polyvinylidene fluoride (PVDF) membranes were purchased from Pall Gelman Laboratory (Ann Arbor, MI, USA). NS398 was purchased from Calbiochem (Darmstadt, Germany). Methyl-nonyl ketone was supplied by the National Institute for the Pharmaceutical and Biological Products of China. All reagents used were of analytical grade.
2.2. Animals
All experimental procedures utilizing mice were carried out in accordance with the established National Institute of Health guidelines.
Female Chinese Kun Ming mice weighing 18–20 g were supplied by the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). Animals were housed in standard laboratory conditions at a stable temperature of 22 ± 3 °C, with a relative humidity of 50–55% and a regulated 12 h light/dark cycle. All animals were provided with food and water ad libitum.
2.3. Peritoneal macrophages isolation and cell culture
Peritoneal macrophages were obtained from mice that had been euthanized by cervical dislocation. The peritonea of the animals were surgically exposed using a midline incision. An experimental group of mice had been administered an intraperitoneal injection of 2 ml of 3% (m/v) sodium thioglycollate three days prior to the sacrifice. Following removal of the carotid artery, the excised tissue was immersed in 75% ethanol for 3–5 min; the peritoneal fluid was then harvested by injecting 4 ml of ice-cold PBS into the peritoneal cavity and subsequent syringe aspiration. Cell suspensions were pelleted by centrifugation and washed for two times.
Cells were incubated in RPMI 1640 complete medium supplemented with 10% FBS for 4 h at 37 °C with 5% CO2 in a humidified chamber.
2.4. Preparation of HC extracts
HC was air dried, ground in a high-speed rotary cutting mill, and screened to produce fractions of 150 μm in size. The particle size of the plant powder is known to be an important factor for effective steam distillation, and was carefully controlled for in our study.
The essential oil was purified by steam distillation extraction. Briefly, 100 g of 150 μm particle size sample was placed into a 2000 mL distillation flask, 1000 mL deionized water was added and the mixture was distilled for 4 h. Oil was collected from the condenser, and 0.2 mL of the oil was dried with anhydrous sodium sulfate. For cell culture studies, the resultant essential oil was weighed and dissolved in DMSO to achieve the desired concentrations.
2.5. Prostaglandin E2 (PGE2) release and COX-2 enzyme activity
To determine PGE2 accumulation from endogenous arachidonic acid, the mouse peritoneal macrophages were seeded in 96-well plates (5 × 104/200 μL/well), cultured for 48 h and, after supernatants were replaced by fresh medium, incubated with or without LPS in the absence or presence of the test agents for 24 h. The levels of PGE2 were measured in cell culture supernatants and cell lysates by using the PGE2 enzyme immunometric EIA kit (Cayman Chemical, Ann Arbor, MI, USA).
The COX-2 enzyme activity was indirectly determined by PGE2 levels in LPS-stimulated macrophages. First, in order to irreversibly inactivate COX-1, mouse peritoneal macrophage cells (1 × 105 cells in a 96-well plate) were pretreated with acetylsalicylic acid (250 μM) for 30 min. Thereafter, cells were washed with PBS and fed with fresh medium. Induction of COX-2 was then achieved by adding LPS (1 μg/mL) to the culture media. Twenty-four hours later, the media was aspirated and cells were washed with PBS before supplying with fresh FBS-free media. Test compounds were pre-incubated for 30 min before exogenous arachidonic acid was added. After 15 min, supernatants were removed and analyzed by the PGE2 enzyme immunometric EIA assay. Experiments were performed at least three times in triplicate.
2.6. Cell viability assay
Cell viability was determined by the mitochondrial-dependent reduction of MTT to formazan (Denizot and Lang, 1986). After the supernatants were removed for PGE2 determination, the cells were exposed to MTT (5 mg/mL) and incubated at 37 °C. Forty-five minutes later, the media was aspirated and cells were solubilized in DMSO (250 μL) for at least 2 h in the dark. The extent of reduction of MTT was quantified by spectrophotometric optical density measurement at 490 nm.
2.7. Western blot analysis
Macrophages that had been grown to confluence in 6-well plates were incubated with or without LPS in the absence or presence of the test agents. Cells were washed with ice-cold PBS and stored at −70 °C until further analysis. Frozen plates were thawed on ice and cells were lysed in a solution of 1% Triton X-100, 0.15 M NaCl and 10 mM Tris–HCl, pH 7.4, for 30 min. Lysates were homogenized through a 22 gauge needle and centrifuged at 10,000 × g for 10 min at 4 °C. The supernatants were collected and total protein was measured (Bradford, 1976). Cell lysates containing equal amounts of protein (50 mg) were boiled in SDS sample buffer for 5 min before separation on a 10% SDS-polyacrylamide gel. Proteins were transferred to PVDF membranes. Membranes were blocked with 5% fat-free dry milk in TBS-T, pH 8.0 (Tris-buffered saline [50 mM Tris, pH 8.0, and 150 mM NaCl] with 0.1% Tween 20) and then incubated with a mouse immunoglobulin G1 against either COX-1 or COX-2, or monoclonal anti-β-actin antibody (all 1:500 dilutions), and incubated overnight at 4 °C. After washing three times with TBS-T, COX-1 and COX-2 was visualized by using an anti-mouse IgG: horseradish peroxidase conjugate and the enhanced chemiluminescence system (ECL™; Amersham Pharmacia Biotech, Piscataway, NJ, USA). Signal intensities were evaluated by densitometric analysis (Kodak Digital Science™ Image Station 2000R; Life Science Products, Rochester NY, USA).
2.8. Real time-PCR analysis
Macrophages (5 × 106 cells in a 10 cm dish) were incubated for 24 h with or without various concentrations of HC and LPS (1 μg/mL). After washing with PBS twice, total RNA was isolated from the cell pellet using the RNAfast200 isolation kit (Fastagen Biotech, Shanghai, China) according to the manufacturer's directions. Quantitative real-time PCR was performed (Livak and Schmittgen, 2001) in a Light Cycler instrument (Roche Diagnostics, Mannheim, Germany) with the FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Mannheim, Germany), and results were analyzed with the LDCA software supplied with the machine. Each 50 μL PCR reaction contained 1/50th of the original cDNA synthesis reaction, 7 μL (25 mM) MgCl, 0.8 μL (20 pmol/L) of each primer, 1 μL (10 mM) dNTPs, 1 μL SYBR Green I Mix, 0.5 μL (5U/L) Taq and 5 μL Buffer. A total of 10 × 50 cycles of amplification were performed. The primers for COX-1 were 5′-CAGGAGGTGTTTGGGTTGCTTC-3′ (sense) and 5′-GGATGAGGCGAGTGGTCTGG-3′ (antisense); COX-2 were 5′-GGTGCCTGGTCTGATGATGTATG-3′ (sense) and 5′-ATGAGTATGAGTCTGCTGGTTTGG-3′ (antisense). Those for actin were 5′-GGCACCACACCTTCTACAATGAG-3′ (sense) and 5′-GGCGTGAGGGAGAGCATAGC-3′ (antisense). The PCR amplification was performed under the following conditions: 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s and extension at 72 °C for 45 s, using the Px2 thermal cycler (Thermo Electron Corporation, Waltham, MA, USA). The fluorescence signal was detected at the end of each cycle. Melting curve analysis was used to confirm the specificity of the products.
2.9. Statistical analysis
Data is presented as the mean and standard deviation (SD) of three independent experiments. Statistical analysis was performed with one-way analysis of variance (ANOVA). All analyses were performed using SPSS software (Chicago, IL, USA). A p value <0.05 was considered to be statistically significant.
3. Results
3.1. Effect of HC essential oil on PGE2 accumulation and COX-2 enzyme activity in LPS-induced peritonea macrophages
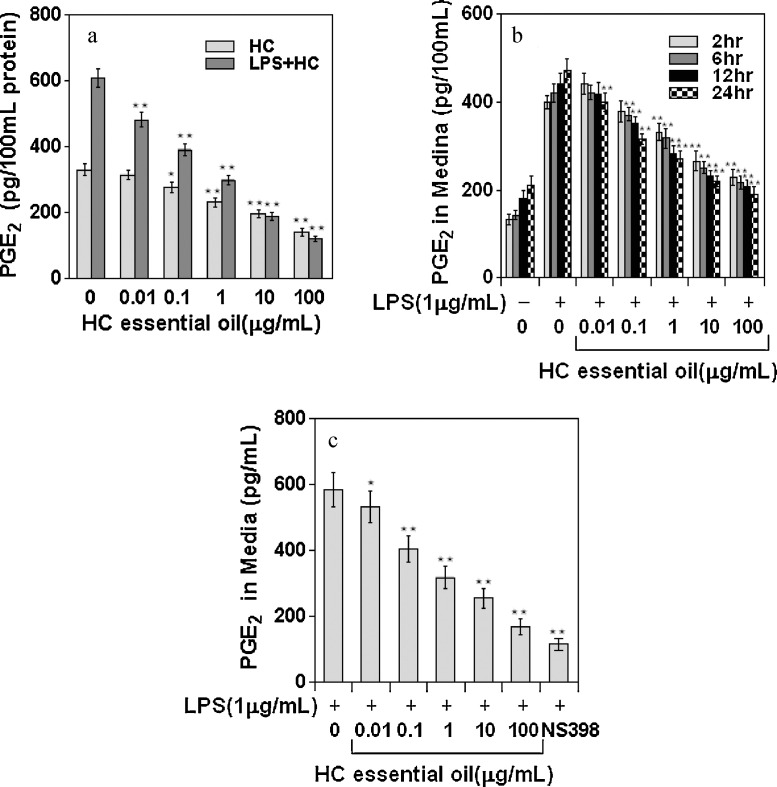
The effect of HC essential oil on inhibition of endogenous prostaglandin E2 levels in peritoneal macrophages was determined. As shown in Fig. 1A, treatment of macrophages with HC essential oil caused a decrease in endogenous PGE2 levels in peritoneal macrophages, which was more pronounced at doses of 10 and 100 μg/mL of HC essential oil. HC essential oil exposure did not affect cell viability up to 100 μg/mL doses, as determined by MTT assay (>95% cell viability; data not shown).
Fig. 1.
Effect of HC essential oil on PGE2 in LPS-induced peritonea macrophages. (A) PGE2 accumulation in cell supernatants in the absence and presence of HC essential oil (0.01–100 μg/mL). (B) Time-dependent accumulation of PGE2 in cell culture media after HC treatment. Bars represent mean ± SD of at least three independent experiments, each performed in triplicate. **p < 0.01. (C) Effect of HC essential oil on COX-2 enzyme activity, as determined by PGE2 levels, in LPS-induced peritonea macrophages. Peritonea macrophages were treated with 0.01–100 μg/mL doses of HC essential oil, in which COX-1 was irreversibly inactivated by acetylsalicylic acid and activated with 1 μg/mL LPS to induce COX-2. The cells were supplemented with fresh culture medium treated with or without HC essential oil or NS398. The reaction was initiated by adding arachadonic acid, and PGE2 levels were measured. Bars represent mean ± SD of at least three independent experiments, each performed in triplicate. *p < 0.05, **p < 0.01.
In the next series of experiments, we examined the effects of LPS challenge on peritoneal macrophages in the presence or absence of HC. LPS exposure is known to cause induction of COX-2 and to convert LPS-induced endogenous arachidonic acid to PGE2. Peritoneal macrophages treated with LPS (1 μg/mL) in the presence of HC essential oil (0.01–100 μg/mL) for 24 h exhibited a dose-dependent decrease in endogenous PGE2 production (Fig. 1A). As shown in Fig. 1B, exposure of cells to HC essential oil caused a time-dependent decrease in PGE2 release into the cell culture medium. The IC50 value was calculated to be 44.8 μg/mL for HC essential oil.
We determined the efficacy of HC towards inhibiting COX-2 activity and compared its effects with those of NS398, an established selective COX-2 inhibitor. Specifically, we evaluated peritonea macrophages in which COX-2 was induced by LPS and exogenous arachidonic acid was added as substrate. As shown in Fig. 1C, HC was able to inhibit the conversion of exogenous arachidonic acid to PGE2 in a dose-dependent manner, which corresponded to inhibition of COX-2 activity. The IC50 value (30.9 μg/mL) was very similar to that determined for the HC-dependent inhibition of PGE2 production in LPS-induced peritonea macrophages.
3.2. Effect of HC on LPS-induced COX-1 and COX-2 expression
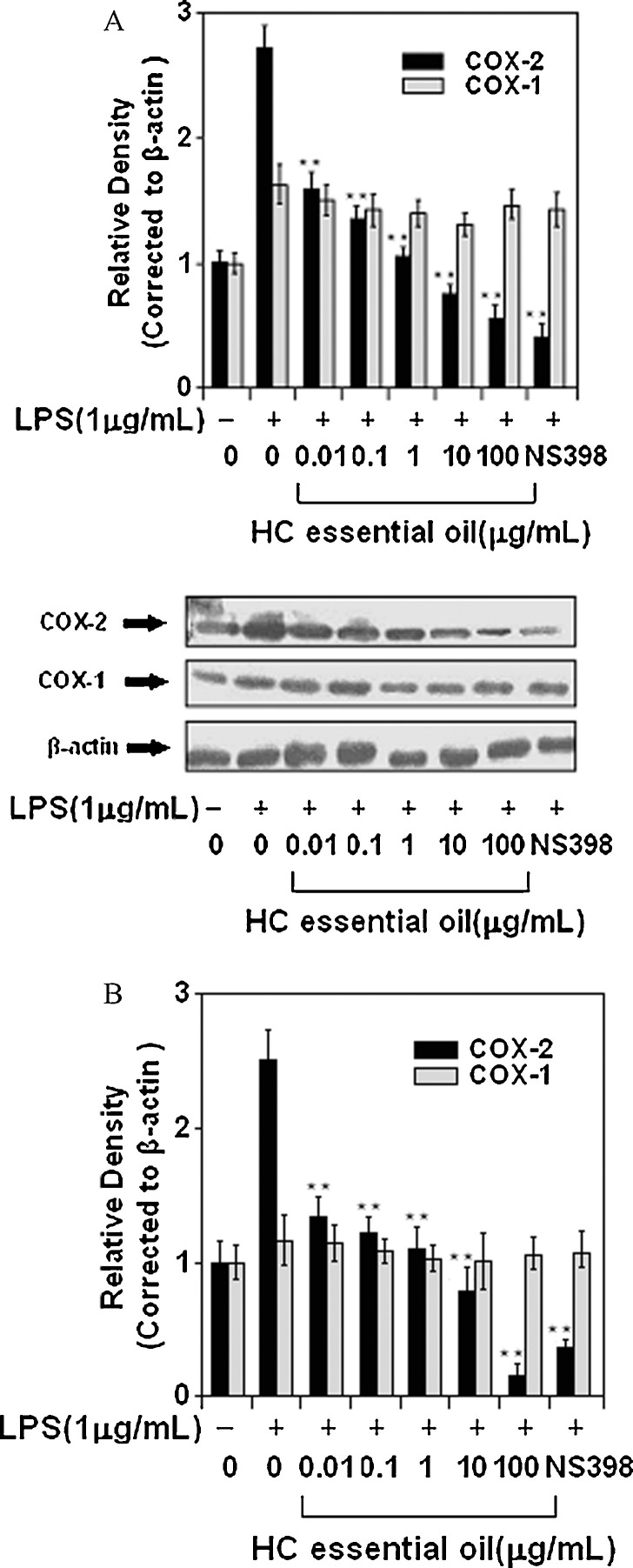
In an attempt to determine the underlying mechanism leading to reduced PGE2 production and release in response to HC exposure, we first examined the influence of HC on LPS-induced COX-2 mRNA levels. NS398 was used as the positive control. As shown in Fig. 2A, COX-2 mRNA levels were significantly elevated after LPS challenge of peritoneal macrophages. A marked decrease in COX-2 mRNA expression was noted after treatment of cells with 10 and 100 μg/mL doses of HC essential oil. Surprisingly, treatment of peritoneal macrophages with test doses of HC essential oil did not cause any significant changes in the COX-1 mRNA levels. Since increased COX-2 mRNA steady state levels may lead to increased COX-2 protein, we examined the influence of HC essential oil on LPS-induced COX-2 protein expression. As shown in Fig. 2B, treatment of cells with doses of 0.01–100 μg/mL HC essential oil caused a significant decrease in COX-2 protein expression, whereas COX-1 protein expression remained unchanged at these doses of HC.
Fig. 2.
Effect of HC essential oil on COX-1 and COX-2 expression in peritonea macrophages. (A) Western blots for COX-1 and COX-2 protein expression, and (B) mRNA expression of COX-1 and COX-2 in peritonea macrophages stimulated with LPS and LPS plus HC essential oil. mRNA and protein levels of COX-1 and COX-2 were normalized to β-actin loading control. Bars represent mean ± SD of at least three independent experiments; the picture is of a single representative experiment. **p < 0.01.
4. Discussion
The present study demonstrated that HC essential oil harbors the ability to inhibit release of PGE2 from LPS-induced mouse peritoneal macrophages (IC50 value: 44.8 μg/mL). The inhibitory activity of HC essential oil was due to a dose-dependent inhibition of COX-2 enzyme activity (IC50 value: 30.9 μg/mL). In addition, HC essential oil was found to reduce COX-2 mRNA and protein expression, but did not affect the activity or expression of COX-1, the constitutively expressed form of cyclooxygenase. HC appeared to inhibit prostaglandin synthesis by a mechanism similar to that induced by NSAIDs. NS398, an NSAID and a specific COX-2 inhibitor, caused a significant decrease in PGE2 levels and also inhibited COX-2 activity and protein expression, but not COX-2 mRNA expression in mouse peritoneal macrophages challenged with LPS.
Numerous studies have demonstrated that the expression of COX-2 in murine peritoneal macrophages is largely regulated by transcriptional activation (Kang et al., 2006, Mestre et al., 2001). Mouse peritoneal macrophages are commonly used for in vitro COX assay due to the convenience of their preparation and harvesting (Lee et al., 1992). Although several other cells are often present in the harvested peritoneal macrophage population, the undesired cells can be easily removed during subsequent washing steps since macrophages stick to the plate. In addition, in mouse peritoneal macrophages a sufficient amount of PGE2 is produced by LPS through the COX-1 or COX-2 pathway (Simmons et al., 2004). Prostaglandins play an important role in the inflammatory process (Hennebert et al., 2008). Therefore, a quantitative analysis of the PGE2 level in mouse peritoneal macrophages can be a good marker of anti-inflammatory activity produced by test substances.
In order to obtain more information about the mechanism of action, the effect of HC in vitro on the COX-2 activities was investigated using mouse macrophages. Inhibition of prostaglandin synthesis by direct interference with the cyclooxygenase enzymes is a common mechanism of NSAIDs. Since HC appeared to have actions similar to those of NSAIDs, we hypothesized that the specific COX-2 inhibitor NS398 might affect PGE2 production and release in LPS-induced mouse peritoneal macrophages in a manner similar to HC. As shown in Fig. 1C, NS398 significantly inhibited endogenous PGE2 production and release in mouse peritoneal macrophages exposed to LPS, similar to that observed in response to HC treatment. The decrease in PGE2 levels after HC treatment corresponded with the decrease in COX-2 mRNA and protein expressions; however, NS398 also reduced mRNA COX-2 levels (Fig. 2B) and COX-2 protein expression ((Fig. 2A) to similar extents as seen with HC. The results of this study partially reinforce the hypothesis that anti-inflammatory effects of HC are related with the inhibition of COX-2 activity, which is frequently enhanced during inflammatory processes (De Leval et al., 2002).
In conclusion, we have demonstrated that the modulation of COX-2 is a possible pathway through which HC may function to prevent various inflammatory responses.
Acknowledgement
This work was supported by a research grant from Xi’an Jiaotong University.
References
- Blobaum A.L., Marnett L.J. Structural and functional basis of cyclooxygenase inhibition. Journal of Medicinal Chemistry. 2007;50:1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang J.S., Chiang L.C., Chen C.C., Liu L.T., Wang K.C., Lin C.C. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. American Journal of Chinese Medicine. 2001;29:303–312. doi: 10.1142/S0192415X01000320. [DOI] [PubMed] [Google Scholar]
- Chen M.I., Wen Y.F., Cheng Y.Y. Gas chromatographic/mass spectrometric analysis of the essential oil of Houttuynia cordata Thunb by using on-column methylation with tetramethylammonium acetate. Journal of Aoac International. 2007;90:60–67. [PubMed] [Google Scholar]
- Chiang L.C., Chang J.S., Chen C.C., Ng L.T., Lin C.C. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. American Journal of Chinese Medicine. 2003;31:355–362. doi: 10.1142/S0192415X03001090. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Cheng H.Y., Chen C.C., Lin C.C. In vitro anti-leukemic and antiviral activities of traditionally used medicinal plants in Taiwan. American Journal of Chinese Medicine. 2004;32:695–704. doi: 10.1142/S0192415X04002284. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Venturi N., Spaggiari S. Gastrointestinal safety of novel nonsteroidal anti-inflammatory drugs: selective COX-2 inhibitors and beyond. Acta BioMedica. 2007;78:96–110. [PubMed] [Google Scholar]
- Debabrata M. Selective cyclooxygenase (COX-2) inhibitors and potential risk of cardiovascular events. Biochemical Pharmacology. 2002;63:817–821. doi: 10.1016/s0006-2952(02)00842-0. [DOI] [PubMed] [Google Scholar]
- De Leval X., Julémont F., Delarge J., Sanna V., Pirotte B., Dogné J.-M. Advances in the field of COX-2 inhibition. Expert Opinion on Therapeutic Patents. 2002;12:969–989. [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunology. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Guastadisegni C., Nicolini A., Balduzzi M., Ajmone-Cat M., Minghetti L. Modulation of PGE2 and TNF-γ by nitric oxide in resting and LPS-activated RAW 264.7 cells. Cytokine. 2002;19:175–180. doi: 10.1006/cyto.2002.1955. [DOI] [PubMed] [Google Scholar]
- Han E.H., Park J.H., Jeong H.G. Houttuynia cordata water extracts suppresses anaphylactic reaction and IgE-mediated allergic response. Drug Metabolism Reviews. 2009;41:40–41. doi: 10.1016/j.fct.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Kamiya M., Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on Hsv-1, Influenza-Virus, and Hiv. Planta Medica. 1995;61:237–241. doi: 10.1055/s-2006-958063. [DOI] [PubMed] [Google Scholar]
- Hennebert O., Pelissier M.A., Le Mee S., Wülfert E., Morfin R. Antiinflammatory effects and changes in prostaglandin patterns induced by 7-β-hydroxy-epiandrosterone in rats with colitis. Journal of Steroid Biochemistry and Molecular Biology. 2008;110:255–262. doi: 10.1016/j.jsbmb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Jachak S.M. Cyclooxygenase inhibitory natural products: current status. Current Medicinal Chemistry. 2006;13:659–678. doi: 10.2174/092986706776055698. [DOI] [PubMed] [Google Scholar]
- Jack C., Florian O., John A.B., Powel H.B., John B., Peter G., Janusz J., Carlo L.V., Frank M., Hans J.S., Michael T. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. The Lancet Oncology. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- Ji K.M., Chen J.J., Li M., Liu Z.G., Xia L.X., Wang C.B., Zhan Z.K., Wu X.L. Comments on serious anaphylaxis caused by nine Chinese herbal injections used to treat common colds and upper respiratory tract infections. Regulatory Toxicology and Pharmacology. 2009;55:134–138. doi: 10.1016/j.yrtph.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.J., Wingerd B.A., Arakawa T., Smith W.L. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. Journal of Immunology. 2006;177:8111–8122. doi: 10.4049/jimmunol.177.11.8111. [DOI] [PubMed] [Google Scholar]
- Kwon K.B., Kim E.K., Shin B.C., Seo E.A., Yang J.Y., Ryu D.G. Herba houttuyniae extract induces apoptotic death of human promyelocytic leukemia cells via caspase activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Experimental and Molecular Medicine. 2003;35:91–97. doi: 10.1038/emm.2003.13. [DOI] [PubMed] [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S.F., Lau C.P., Ho H.M., Lee M.Y.H., Au S.W.N., Cheng C.H.K., Lau C.B.S., Tsui S.K.W., Wan D.C.C., Waye K.P., Wong M.M.Y., Wong K.B., Lam C.K., Leung C.W.K., Fung P.C. Immunomodulatory and anti-SARS activities of Houttuynia cordata. Journal of Ethnopharmacology. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. Journal of Biological Chemistry. 1992;267:25934–25938. [PubMed] [Google Scholar]
- Li G.Z., Chai O.H., Lee M.S., Han E.H., Kim H.T., Song C.H. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biological & Pharmaceutical Bulletin. 2005;28:1864–1868. doi: 10.1248/bpb.28.1864. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lin T.Y., Liu Y.C., Jheng J.R., Tsai H.P., Jan J.T., Wong W.R., Horng J.T. Anti-enterovirus 71 activity screening of Chinese herbs with anti-infection and inflammation activities. American Journal of Chinese Medicine. 2009;37:143–158. doi: 10.1142/S0192415X09006734. [DOI] [PubMed] [Google Scholar]
- Loren M.D. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin Arthritis Rheum. 2002;32:25–32. doi: 10.1053/sarh.2002.37217. [DOI] [PubMed] [Google Scholar]
- Lu H.M., Liang Y.Z., Chen S. Identification and quality assessment of Houttuynia cordata injection using GC–MS fingerprint: a standardization approach. Journal of Ethnopharmacology. 2006;105:436–440. doi: 10.1016/j.jep.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Lu H.M., Liang Y.Z., Yi L.Z., Wu X.J. Anti-inflammatory effect of Houttuynia cordata injection. Journal of Ethnopharmacology. 2006;104:245–249. doi: 10.1016/j.jep.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre J.R., Rivadeneira D.E., Mackrell P.J., Duff M., Stapleton P.P., Mack-Strong V., Maddali S., Smyth G.P., Tanabe T., Daly J.M. Overlapping CRE and E-box promoter elements can independently regulate COX-2 gene transcription in macrophages. FEBS Letters. 2001;496:147–151. doi: 10.1016/s0014-5793(01)02422-x. [DOI] [PubMed] [Google Scholar]
- Mitchell J.M., Akarasereenont P., Thiemebermann C., Flower R.J., Vane J.R. Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proceedings of the National Academy of Sciences. 1994;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuengchamnong N., Krittasilp K., Ingkaninan K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC-ESI-MS coupled with DPPH assay. Food Chemistry. 2009;117:750–756. [Google Scholar]
- Ortiz E. Market withdrawal of Vioxx: is it time to rethink the use of COX-2 inhibitors? Journal of Managed Care Pharmacy. 2004;10:551–554. doi: 10.18553/jmcp.2004.10.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Antiinflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. American Journal of Chinese Medicine. 2005;33:415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Shan Z., Vasan Y., William G.N., William B.I., Angelo M.D.M. Cyclooxygenases in cancer: progress and perspective. Cancer Letters. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Simmons D.L., Botting R.M., Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacology Reviews. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Tang Y.J., Yang J.S., Lin C.F., Shyu W.C., Tsuzuki M., Lu C.C., Chen Y.F., Lai K.C. Houttuynia cordata Thunb extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncology Reports. 2009;22:1051–1056. doi: 10.3892/or_00000535. [DOI] [PubMed] [Google Scholar]
- Thun M.J., Blackard B. Pharmacologic effects of NSAIDs and implications for the risks and benefits of long-term prophylactic use of aspirin to prevent cancer. Recent Results in Cancer Research. 2009;181:215–221. doi: 10.1007/978-3-540-69297-3_20. [DOI] [PubMed] [Google Scholar]
- Vane J.R., Botting R.M. Anti-inflammatory drugs and their mechanism of action. Inflammation Research. 1998;47(Suppl. 2):S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- Xu C.J., Liang Y.Z., Chau F.T. Identification of essential components of Houttuynia cordata by gas chromatography/mass spectrometry and the integrated chemometric approach. Talanta. 2005;68:108–115. doi: 10.1016/j.talanta.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen D.F. Anticomplementary principles of a Chinese multiherb remedy for the treatment and prevention of SARS. Journal of Ethnopharmacology. 2008;117:351–361. doi: 10.1016/j.jep.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]