Highlights
-
•
Total flavonoids of S. tonkinensis showed obvious anticomplement activity after incubated with human intestinal bacteria.
-
•
Eighteen flavonoids were identified from TFST and its metabolites by UPLC-ESI- LTQ/MS.
-
•
Five glycosides were metabolized into three aglycones with more potent anticomplement activity.
Keywords: Flavonoids, Sophora tonkinensis, Intestinal bacteria, Metabolite, Anticomplement
Abstract
Anticomplement activity played an important role in anti-inflammatory effects of traditional Chinese herbs. The total flavonoids of Sophora tonkinensis (TFST) were inactive on the complement system but showed obvious anticomplement activity after incubated with human intestinal bacteria in vitro. In order to discover the metabolic activation of TFST by intestinal flora, the constituents of TFST and its metabolites were identified by UPLC-ESI-LTQ/MS. Their anticomplement activities were evaluated through the classical and alternative pathway. As a result, eighteen flavonoids were identified, including seven flavonoid glycosides, five aglycones and six isoprenylated flavonoids. All the glycosides (daidzein-4′-glucoside-rhamnoside, sophorabioside, rutin, isoquercitrin, quercitrin, ononin, trifolirhizin) were metabolized into their corresponding aglycones in different extent by human intestinal bacteria, resulting in the contents of the five aglycones were highly increased in 24 h. However, no changes have occurred on the six isoprenylated flavonoids. Interestingly, three aglycones (quercetin, formononetin and maackiain) had significantly more potent anticomplement activities than their prototype glycosides. The results indicated that the enhancement of TFST anticomplement activity was attributed to the active aglycones, especially formononetin and quercetin, produced by human intestinal bacteria. These aglycones are likely to be among the potential active components of S. tonkinensis for its inhibiting inflammation effects.
1. Introduction
The roots and rhizomes of Sophora tonkinensis, known as “Shandougen” in traditional Chinese medicine, have been recorded in Chinese Pharmacopoeia and widely used for the treatment of laryngopharyngeal inflammation and throat pain through oral administration [1,2]. Flavonoids and alkaloids are the two kinds of major components of S. tonkinensis. Pharmacological studies reported that the flavonoids in S. tonkinensis possessed significant bioactivities, such as antioxidant, anticancer, anti-gastric ulcer, and inhibiting gastric acid secretion effects [[3], [4], [5], [6]]. The alkaloids of S. tonkinensis exhibited antiviral activity against hepatitis B virus (HBV), influenza virus A/Hanfang/359/95 (H3N2) and coxsackie virus B3 (CVB3) [7,8].
Most traditional Chinese medicines are given orally and the chemical compounds inevitably contact intestinal flora within the gastrointestinal tract. Moreover, those components were metabolized by intestinal bacteria before absorbed from the digestive tract [9,10]. Research on herbal components of metabolism with intestinal flora is greatly significant to understand their potential biological characteristics [11].
The system of complement plays a dominant role in regulating host defense. However, the pathogenesis of some diseases, such as severe acute respiratory syndrome (SARS) and acute respiratory distress syndrome (ARDS), may cause the complement system excessive activation and lead to systemic inflammation response [12,13]. It’s notable that, traditional Chinese medicine commonly had beneficial effects on infectious diseases by inhibiting inflammation and reducing fever, and the mechanism is probably related to inhibiting excessive activation of the complement system in vivo [14,15]. Hence, the discovery and application of complement inhibitors should become a potential therapeutic strategy for the treatment of these infectious diseases.
Interestingly, our previous study found that the total flavonoids of S. tonkinensis (TFST) were absence of complement inhibitory activity but showed obvious anticomplement activity after incubated with human intestinal bacteria. However, intestinal bacteria had no distinct influence on the weak anticomplement activity of the total alkaloids of S. tonkinensis. We speculated that TFST might be the key effective components that exhibit anticomplement activity in vivo after metabolized with intestinal bacteria.
To find out how the structures and activities of TFST were changed by human intestinal bacteria, TFST was extracted and incubated with human fecal microflora in this study. Components of TFST and their metabolites were recognized and compared via UPLC-ESI-LTQ/MS, as well as their anticomplement activities in vitro.
2. Experimental
2.1. Materials and reagents
The dried roots of S. tonkinensis were purchased from Leiyunshang drug store (Shanghai), and authenticated by Prof. DaoFeng Chen at Fudan University. The voucher specimen (SDG-JX07) has been deposited at School of Pharmacy, Fudan University, Shanghai, People’s Republic of China.
Sheep blood cells were prepared in Alsevers’ solution. Anti-sheep erythrocyte antibody was provided by Prof. Yunyi Zhang. Guinea pig serum was prepared by healthy guinea pigs and rabbit blood cells were prepared from the ear vein of New Zealand white rabbits (both purchased from Laboratory Animals Research Institute of Fudan University, Shanghai, China.). Normal human serum (NHS) was prepared from healthy male donors (at the age of 20–30 years old). Buffers: VBS2+, isotonic veronal buffered saline, containing 0.15 mmol/L Ca2+ and 0.5 mmol/L Mg2+. EGTA-VBS2+, veronal buffer saline, containing 5 mmol/L Mg2+ and 8 mmol/L ethylene glycol tetraacetic acid.
Reference substances (purity>98 %): ononin, formononetin, trifolirhizin, maackiain, rutin, quercitrin, isoquercitrin, quercetin, daidzein, genistein and sophorabioside were purchased from Meilun Biological Technology Co., Ltd (Shanghai, China). UPLC grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Ultrapure water was purified by the Milli-Q water purification system from Millipore (Boston, MA, USA). General anaerobic medium broth (GAM broth) was purchased from Meilun Biological Technology Co., Ltd (Shanghai, China). All other reagents and chemical compounds were of analytical grade.
2.2. UPLC-MS analysis
Analyses of TFST and its metabolites were performed using a UPLC system (Dionex ultimate 3000) with a conditioned autosampler at 4 °C. The qualitative analysis and separation were performed on a C18 column (4.6 × 250 mm, i.d., 5 μm Thermo, Finnigan, San Jose, CA, USA). The temperature of the column was set at 25 °C using an UPLC system (Thermo, Finnigan, San Jose, CA, USA) The mobile phase consisted of 0.1 % formic acid add in ultra-pure water (A, v/v) and acetonitrile (B) using a gradient elution of 6–20 % B at 0−20 min, 20–90 % B at 20−80 min, and the flow rate was 0.5 mL/min. The UV absorption was set at 310 nm and the injection volume was 5 μL. The mass spectra were set in the range of m/z 110-1500. The electrospray ionization (ESI) source was acquired in negative ion mode with the ion spray voltage at 3.5 Kv; the temperature of the capillary was maintained at 350 °C. Nitrogen was used as sheath gas and auxiliary gas with a flow rate of 30 arbitrary units and 10 arbitrary units, respectively. The helium was used as the damping gas and collision gas [2].
2.3. Preparation of total flavonoids
Dried roots of S. tonkinensis (50 g) were extracted three times using 80 % ethanol for 2 h by heating reflux method, after filtrated, the filtrate was collected and evaporated in vacuum. The 80 % ethanol extract was mixed in water and purified with an AB-8 macroporous resin column, eluted successively with distilled water 3 times column volume and then use 80 % ethanol to wash 6–8 times column volume [16]. 80 % ethanol elution was collected and evaporated in vacuum to yield TFST (4.18 g).
2.4. Metabolism of flavonoid samples by human intestinal bacteria in vitro
Fresh fecal samples were obtained from 8 healthy human volunteers (four males, four females, 20–40 years of age, had neither gastrointestinal disease history nor use of antibiotics for at least six months before the experiment), and homogenized with 20 times volume of general anaerobic medium broth immediately [17]. After removing residue using gauze filter, the intestinal bacterial suspension was cultured in an anaerobic system (nitrogen 85 %, carbon dioxide 10 %, hydrogen 5%) at 37 °C. 20 mg TFST was added to 20 mL human intestinal bacterial suspension and the mixture was anaerobically incubated at 37 °C for 24 h. The incubated solution was respectively taken out and extracted with water saturated n-butanol three times. The extract was centrifuged at 6000 rpm for 10 min at 4 °C. The supernatant was evaporated in vacuum to dryness. The residue was dissolved in 1 mL MeOH and centrifuged at 14,000 rpm for 8 min at 4 °C. The supernatant was then analyzed by UPLC-ESI-LTQ/MS. The chemical constituent change in TFST was also analyzed at incubation time points of 0, 12 and 24 h.
Using the same method as above, 5 mg of each reference substance (sophorabioside, ononin, trifolirhizin, rutin, quercitrin and isoquercitrin) was added in 10 mL human intestinal bacterial suspension and incubated anaerobically for 24 h at 37 °C. After extracted by n-butanol and purified through centrifugation, the final methanol solution was also analyzed using UPLC-ESI-LTQ/MS.
2.5. Anticomplement activity assay
The anticomplement activities through the classical pathway of sophorabioside, rutin, isoquercitrin, quercitrin, ononin, trifolirhizin, daidzein, genistein, quercetin, formononetin, maackiain and heparin sodium (positive control) were evaluated according to Mayer's modified method [18]. Their anticomplement activities through the alternative pathway were measured according to Klerx's method [19].
2.6. Anti-inflammatory activity assay
To evaluate the effect of sophorabioside, rutin, isoquercitrin, quercitrin, ononin, trifolirhizin, daidzein, genistein, quercetin, formononetin, maackiain on NO secretion, lipopolysaccharide (LPS) was used as initiators in RAW264.7 cells. The murine macrophage cell line RAW264.7 was maintained in DMEM (Gibco, NY, USA) containing 10 % (v/v) fetal bovine serum (Gibco) and cultured in 96-well plates (1 × 105 cells per well), at 37 °C in a humidified atmosphere with 5% CO2. LPS (10 ng/ml) and test samples were added to the corresponding wells and incubated for 24 h. The NO content in the culture media was measured according to the colorimetric method, using Griess reagent [16].
3. Results and discussion
3.1. Biotransformation of TFST by human intestinal bacteria in vitro
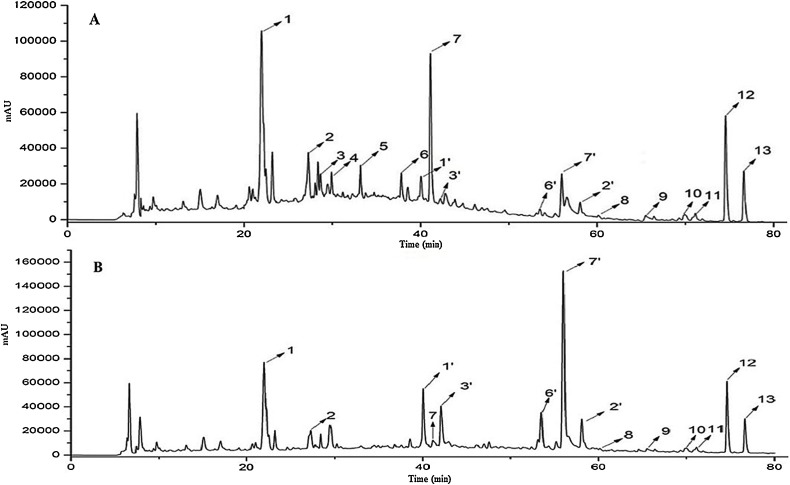
Fig. 1 presents the UPLC chromatograms of TFST and its metabolites obtained by incubating with the human intestinal bacterial mixture for 24 h. The results showed that no obvious new peak in the metabolites (Fig. 1B) was observed compared with TFST (Fig. 1A), but the peak height and peak area changed significantly.
Fig. 1.
UPLC chromatograms of TFST (A) and its metabolites produced by human intestinal bacteria (B).
3.2. Identification constituents of flavonoids
The chromatographic peaks 2–7, 1′, 2′, 3′, 6′ and 7′ of TFST, were identified by comparing the retention times, molecular ion peaks and UV spectra with reference substances by UPLC-ESI-LTQ/MS in negative ionization mode. The data were summarized in Table 1 . The negative electrospray mass spectra of peaks 2–7 revealed [M-H]− ions at m/z 577.06, m/z 609.12, m/z 463.07, m/z 447.09, m/z 429.08 and m/z 445.08 were identified as six flavonoid glycosides, including sophorabioside, rutin, isoquercitrin, quercitrin, ononin and trifolirhizin. The peaks 1′, 2′, 3′, 6′ and 7′ revealed [M-H]− ions at m/z 252.95, m/z 268.96, m/z 300.94, m/z 266.99 and m/z 283.09, and were identified as five flavonoid aglycones, including daidzein, genistein, quercetin, formononetin and maackiain.
Table 1.
Retention time (RT), molecular ion peaks and peak area ratios of 7 flavonoid glycosides and 5 aglycones in TFST and its metabolites.
| NO. | Compound | RT (min) | [M-H]− m/z | Molecular Formula | Area (%) in TFST/Area (%) after incubation |
|---|---|---|---|---|---|
| 1 | Daidzein-4′- glucoside- rhamnoside |
22.48 | 561.09 | C27H30O13 | 19.47/14.21 |
| 2 | Sophorabioside | 27.74 | 577.06 | C27H30O14 | 9.51/5.17 |
| 3 | Rutin | 28.91 | 609.12 | C27H30O16 | 3.17/0 |
| 4 | Isoquercitrin | 30.23 | 463.07 | C21H20O12 | 3.78/0 |
| 5 | Quercitrin | 33.56 | 447.09 | C21H20O11 | 5.74/0 |
| 6 | Ononin | 38.17 | 429.08 | C22H22O9 | 5.89/0 |
| 7 | Trifolirhizin | 41.81 | 445.08 | C22H22O10 | 17.24/0 |
| 1′ | Daidzein | 40.57 | 252.95 | C15H10O4 | 3.21/9.74 |
| 2′ | Genistein | 59.19 | 268.96 | C15H10O5 | 1.75/7.16 |
| 3′ | Quercetin | 42.24 | 300.94 | C15H10O7 | 0.17/9.96 |
| 6′ | Formononetin | 54.63 | 266.99 | C16H12O4 | 0.21/7.74 |
| 7′ | Maackiain | 56.37 | 283.09 | C16H12O5 | 6.21/38.47 |
Compound 1 gave a [M-H] − ion at m/z 561.09 and fragment ions at m/z 414.97, 396.92, 378.96 and 349.01. The ion at m/z 414.97 was corresponding to the loss of rhamnose [M-H-rha]−, the ions at m/z 396.92 and 378.96 were generated by losing one and two molecules of H2O (18 Da), the fragment ion at m/z 349.01 was corresponding to [M-H-rha-2H2O-2CH3]-. The fragmentation pathways were similar to those of compound 2, except for 16 Da (-OH) less than every fragment ion of compound 2. Besides, considering the molecular weight of compound 1 was just 308 DA (-glc-rha) more than that of 1′, we speculated that compound 1 and 2 have the same metabolic pathways. Thus, compound 1 was identified as daidzein-4′-glucoside-rhamnoside.
Compounds 8–13 showed typical fragmentation patterns of isoprenylated flavonoids, which could be distinguished by the characteristic 1,3A-ion and 1,3B ions according to the Retro Diels Alder (RDA) Cleavage (Fig. S1) and the characteristic fragments originated from the degradation of different isopentene groups in the MS/MS spectra (Fig. S2). The results were summarized in Table 2 .
Table 2.
Characterization of isoprenylated flavonoids of S. tonkinensis.
| NO. | Identification | RT (min) | [M-H]− m/z |
Molecular Formula | MS/MS |
|---|---|---|---|---|---|
| 8 | Kurarinone | 60.15 | 437.15 | C26H32O6 | 419.10[M-H-H2O]−, 313.17[M-H-lavandulyl]−, 300.98(1,3A−), 176.96(1,3A−lavandulyl) |
| 9 | Sophoradin | 66.48 | 459.09 | C30H36O4 | 403.13[M-H-isopentenyl]−, 205.21(1,3A-), 204.07[1,3A−-H]−, 176.12[1,3A−-H-CO]−, 149.04(1,3A−-isopentenyl) |
| 10 | Sophoradochromene | 69.82 | 457.03 | C30H34O4 | 401.08[M-H-isopentenyl]−, 253.08(1,3B−), 205.13(1,3A−), 204.09[1,3A−-H]−, 176.05[1,3A−-H-CO]−, 149.22(1,3A−-isopentenyl) |
| 11 | 2-(3-Hydroxy-2,2-dimethyl-8-prenyl-6-chromanyl)-7-hydroxy-8-prenyl-4-chromanone | 71.25 | 475.14 | C30H36O5 | 419.15[M-H-isopentenyl]−, 269.21(1,3B−), 203.11(1,3A−), 147.14(1,3A−-isopentenyl) |
| 12 | Sophoranone | 77.49 | 459.05 | C30H36O4 | 403.08[M-H-isopentenyl]−, 255.19 (1,3B−), 203.14(1,3A−), 147.06(1,3A−-isopentenyl) |
| 13 | Sophoranochromene | 78.26 | 457.1 | C30H34O4 | 401.21[M-H- isopentenyl]−, 253.11(1,3B−), 203.13(1,3A−), 147.06(1,3A−-isopentenyl) |
Compound 8 gave the [M-H]− ion at m/z 437.15, and the fragment ions at m/z 419.10, 313.17, 300.98, and 176.96, which were the typical fragmentation pattern of isoprenylated flavonoid [20]. The ion at m/z 419.10 was generated by losing a molecule of water and the ion at m/z 313.17 was corresponding to the loss of lavandulyl group in
a similar pathway. The ion at m/z 300.98 (1,3A-) was attributed to RDA cleavage of flavanone and the ion at m/z 176.96 was corresponding to the loss of lavandulyl group (1,3A-- lavandulyl) sequentially. By matching experimental MS/MS spectra with those reported in the literature [21], compounds 8 was identified as kurarinone.
Compound 9 showed the [M-H]− ion at m/z 459.09, and the fragment ions at m/z 403.13, 205.21, 204.07, 176.05 and 149.04, which were corresponding to [M-H- isopentenyl]-, (1,3A-), [1,3A--H]-, [1,3A--H-CO]− and (1,3A--isopentenyl) respectively. The fragmentation pathways were consistent with sophoradin [22].
Compound 10 gave the [M-H]− ion at m/z 457.03, the fragment ions at m/z 401.08, 253.08, 205.13, 204.09, 176.05 and 149.22, which were the typical fragmentation pattern of isoprenylated flavonoid. The ion at m/z 401.08 was corresponding to the loss of isopentene group. The ions at m/z 253.08 (1,3B-) and 205.13 (1,3A-) were attributed to RDA Cleavage of flavanone and the ion at m/z 149.22 was corresponding to the loss of isopentene group (1,3A--isopentenyl) sequentially. Based on MS/MS spectra with those reported in literature and database [23], compounds 10 was identified as sophoradochromene.
Compounds 11, 12 and 13 showed the [M-H]− ions at m/z 475.14, 459.05 and 457.11, the fragment ions at m/z 419.15, 269.21, 203.11, 147.14; 403.08, 255.19, 203.14, 147.06 and 401.21, 253.11, 203.13, 147.06, which were corresponding to [M-H-isopentenyl]−, (1,3B-), (1,3A-) and (1,3A--isopentenyl), respectively. The fragmentation pathways of these three compounds were consistent with 2-(3-Hydroxy-2, 2-dimethyl-8-prenyl-6-chromanyl)-7-hydroxy-8-prenyl-4-chromanone, sophoranone and sophoranochromene [[23], [24], [25], [26]].
In total, eighteen compounds of TFST and its metabolites were identified (Fig. S3), including seven flavonoid glycosides: daidzein-4′-glucoside-rhamnoside (1), sophorabioside (2), rutin (3), isoquercitrin (4), quercitrin (5), ononin (6) and trifolirhizin(7); five flavonoid aglycones: daidzein (1′), genistein (2′), quercetin (3′), formononetin (4′), maackiain (5′) and six isoprenylated flavonoids: kurarinone (8), sophoradin (9), sophoradochromene (10), 2-(3-Hydroxy-2, 2-dimethyl-8-isopentenyl-6-chromanyl)-7-hydroxy-8-isopentenyl-4-chromanone (11), sophoranone (12) and sophoranochromene (13). The area ratio of these peaks in TFST and its metabolites (Table 1) indicated that the flavonoid glycosides were decreased, the flavonoid aglycones were increased by human intestinal bacteria, while the isoprenylated flavonoids were not changed (Fig. 1, Table 2).
3.3. The content changes of ingredients in TFST by human intestinal bacteria
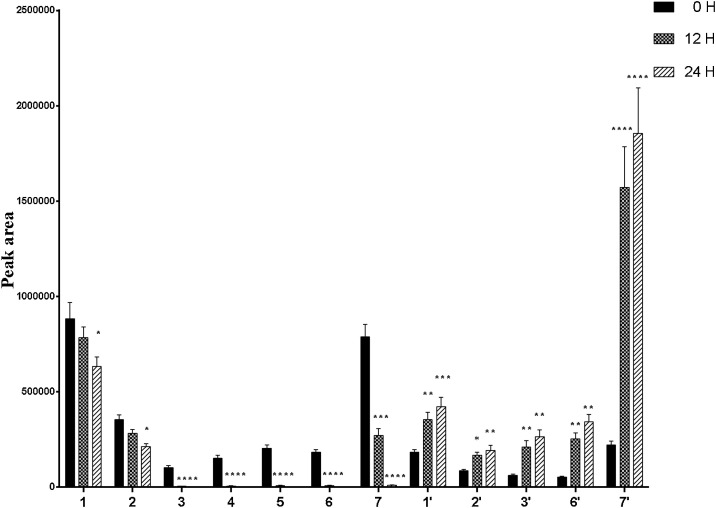
Content changes of the seven flavonoid glycosides and five flavonoid aglycones in TFST and its metabolites were determined semi quantitatively by peak area of each incubation time point (0, 12 and 24 h) to 0 h (100 %) (Table S1). Regression equations, correlation coefficients, precision and LOD of reference standards were also evaluated (Table S2). All the contents of daidzein-4′-glucoside-rhamnoside (1), sophorabioside (2), rutin (3), isoquercitrin (4), quercitrin (5), ononin (6), trifolirhizin (7) declined as time went on, which were exactly opposite to the content changes of daidzein (1′), genistein (2′), quercetin (3′), formononetin (6′), maackiain (7′) (Fig. 2 ). Based on the content changes as well as the chemical structures of the twelve constituents of TFST, we inferred that seven flavone glycosides were converted to their aglycones accordingly by human intestinal bacteria.
Fig. 2.
The Peak area changes of the main constituents in flavonoids of S. tonkinensis when incubated with human intestinal bacteria. Date are presented as mean ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, compared with the initial peak area of the compound.
3.4. Metabolism of flavonoids by human intestinal bacteria in vitro
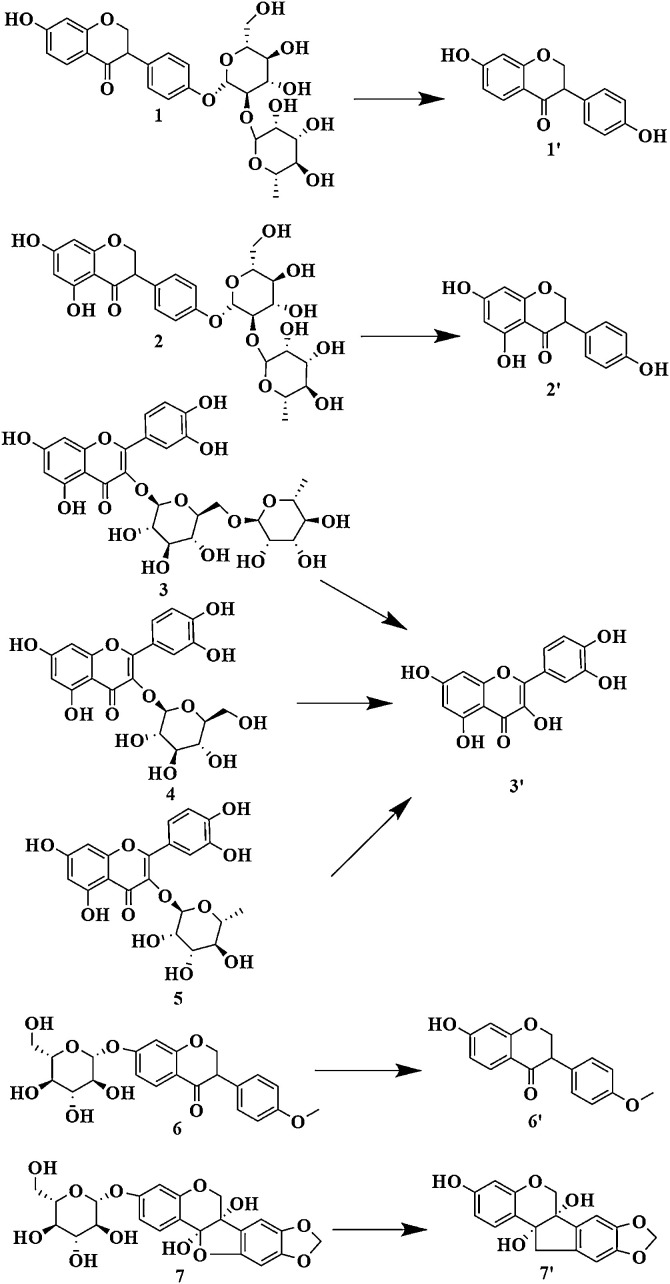
The UPLC chromatograms of reference standards showed the metabolic results in Fig. S4, which confirmed the transformation of the glycosides to their respective aglycones by intestinal bacteria. Based on the above results, the metabolic pathways of flavonoids with human intestinal bacteria were shown in Fig. 3 . It was noteworthy that rutin, isoquercitrin, and quercitrin were all metabolized to quercetin. And since there was no commercially available reference standard of daidzein-4′-glucoside-rhamnoside (1), it was hard to verify metabolite directly through the conversion reference product with human intestinal flora. According to chemical structure, fragmentation patterns and the metabolic result of sophorabioside, we speculated that daidzein-4′-glucoside-rhamnoside was metabolized to daidzein by human intestinal bacteria. The results suggested that O-glycoside flavonoids of TFST were easily converted into their corresponding aglycones with intestinal flora.
Fig. 3.
The pathway of flavonoids metabolism with human fecal bacteria.
3.5. Anticomplement activities through the classical pathway and the alternative pathway
In the transformation of S. tonkinensis flavonoids, there were seven components have changed. Among these components, rutin, isoquercitrin, quercitrin, ononin, trifolirhizin were metabolized into quercetin, formononetin and maackiain, with the anticomplement activities were remarkable improved. Particularly, formononetin (CH50: 0.082 ± 0.029 mg/mL, AP50: 0.217 ± 0.068 mg/mL), the metabolite of ononin (CH50: 3.275 ± 1.352 mg/mL, AP50: NE) showed significant activity comparable to the positive inhibitor. However, neither glycosides nor aglycones of the other isoflavones (sophorabioside, daidzein and genistein) showed anticomplement activity (Table 3 ). Although rutin, isoquercitrin and quercitrin demonstrated anticomplement activities through the classical pathway or alternative pathway, their common metabolite quercetin (CH50: 0.178 ± 0.102 mg/mL, AP50: 0.205 ± 0.139 mg/mL) was quite more potent. Besides, the isoprenylated flavonoids were all inactive.
Table 3.
Anticomplement activities of 6 flavonoids and their metabolites. (means ± S.D., n = 3. *P < 0.05, **P < 0.01, ****P < 0.0001, compared with corresponding flavonoid glycosides.).
| No. | Compound | CH50 (mg/mL) | AP50(mg/mL) |
|---|---|---|---|
| 2 | Sophorabioside | NE | NE |
| 3 | Rutin | 0.247 ± 0.133 | 0.307 ± 0.089 |
| 4 | Isoquercitrin | 0.419 ± 0.187 | 0.296 ± 0.103 |
| 5 | Quercitrin | 0.276 ± 0.172 | 0.305 ± 0.164 |
| 6 | Ononin | 3.275 ± 1.352 | NE |
| 7 | Trifolirhizin | 3.517 ± 1.367 | NE |
| 1′ | Daidzein | NE | NE |
| 2′ | Genistein | NE | NE |
| 3′ | Quercetin | 0.178 ± 0.102*(vs· 3);** (vs· 4);* (vs· 5) | 0.205 ± 0.139*(vs· 3); * (vs· 5) |
| 6′ | Formononetin | 0.082 ± 0.029 ****(vs. 6) | 0.217 ± 0.068 |
| 7′ | Maackiain | 1.949 ± 0.683 *(vs. 7) | 2.522 ± 0.710 |
| Positive control | Heparin | 0.046 ± 0.009 | 0.112 ± 0.019 |
3.6. Anti-inflammatory activities
In vitro anti-inflammatory assays (Fig. S5) showed that compared with cell + LPS group, rutin, quercitrin, quercetin and formononetin treated cells exhibited significant decreased NO production (P < 0.05). On the other hand, the results were consistent with their anticomplement activities. Quercetin and formononetin showed better activities than their glycosides (rutin, isoquercitrin and ononin). Meanwhile, there was no statistical difference between quercitrin and quercetin. However, both maackiain and trifolirhizin, with very weak anticomplement activity, had no anti-inflammatory activity.
The above results revealed that the metabolic activation of TFST by human intestinal flora was attributed to the content increasing of the more potent flavonol (quercetin) and isoflavone (formononetin) aglycones. This kind of transformation had also occurred in the intestinal bacteria metabolism of the inactive flavone glycosides of Scutellaria baicalensis roots to their anticomplement aglycones both in vitro and in vivo [27]. These aglycones, like quercetin and formononetin, might be the potential effective substances of S. tonkinensis to perform the inhibiting inflammation action in vivo.
Our previous study showed that bacteroidetes, proteobacteria and firmicutes accounted for more than 90 % of human intestinal bacteria [27]. Ononin and quercitrin were further metabolized by specific human intestinal bacteria from these three groups, including Bacteroides, Escherichia coli, Lactobacillus and Enterococcus faecalis. The results showed that ononin could be transformed by all the four bacteria into formononetin (Fig. S6). However, only Bacteroides could convert quercitrin to quercetin (Fig. S7). The reason might be the different glycosidic bonds of the two compounds. The β-glucoside in ononin is more common in nature than the α-rhamnoside in quercitrin and thus could be metabolized more easily by most intestinal bacteria. On the other hand, the four bacteria had quite differnt efficinecies on metabolizing ononin, in which Enterococcus faecalis transformed almost all the compound into its aglycone.
Besides, the roots of sophora flavescens Ait, another commonly used medicinal plant from Sophora, are found to contain similar chemical constituents as S. tonkinensis, including isoprenylated flavonoids and quinolizidine alkaloids. However, their clinical applications are completely different [28,29]. S. tonkinensis is frequently used internally to treat acute throat infections while S. flavescens is mostly used externally for the treatment of skin diseases, such as eczema and dermatitis [30]. And their different flavonoid constituents could serve as the qualitative markers for identification of S. tonkinensis and S. flavescens. S. flavescens roots barely contain flavonoid glycosides but mainly contain isoprenylated flavonoids (Fig. S8), which are inactive on the complement system and unable to be metabolized by intestinal bacteria. As expected, almost no change was detected when the flavonoids of S. flavescens were incubated with human intestinal bacteria, except trifolirhizin (1) metabolized to maackiain (1′) (Fig. S9, Table S3). These results further explain the different effects and administration routes of S. flavescens and S. tonkinensis.
4. Conclusion
Our current study showed that the presence of intestinal flora played a key role in metabolic activation of TFST after oral administration. The contents of flavonoid aglycones with potent anticomplement activity were enhanced significantly under the hydrolytic action of intestinal flora on the flavonoid glycosides. Among the eighteen flavonoids identified using UPLC-ESI-LTQ/MS method, isoflavone and flavonol aglycones (formononetin and quercetin) and three flavonol glycosides (rutin, isoquercitrin and quercitrin) were the major anticomplement constituents of TFST and should be taken as the quality markers of S. tonkinensis roots. The in vivo anti-inflammatory and anticomplement effects of formononetin, the most potent constitent and metabolite of TFST, will be investigated in the near future.
CRediT authorship contribution statement
Xin Jin: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing. Yan Lu: Writing - review & editing. Shaoxin Chen: Project administration. Daofeng Chen: Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2019YFC1711000), National Natural Science Foundation of China (81330089) and the Development Project of Shanghai Peak Disciplines-Integrative Medicine (20180101).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jpba.2020.113176.
Contributor Information
Shaoxin Chen, Email: sxzlb@263.net.
Daofeng Chen, Email: dfchen@shmu.edu.cn.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.The State Pharmacopoeia Commission of the People’s Republic of China . vol. 1. China Medical Science Press; Beijing: 2010. (Pharmacopoeia of the People’S Republic of China). P. 25. [Google Scholar]
- 2.He C.M., Cheng Z.H., Chen D.F. Qualitative and quantitative analysis of flavonoids in Sophora tonkinensis by LC/MS and HPLC. Chin. J. Nat. Med. 2013;11:690–698. doi: 10.1016/S1875-5364(13)60081-3. [DOI] [PubMed] [Google Scholar]
- 3.Kajimoto S., Takanashi N., Kajimoto T., Xu M., Cao J., Masuda Y., Aiuchi T., Nakajo S., Ida Y., Nakaya K. Sophoranone, extracted from a traditional Chinese medicine Shan Dou Gen, induces apoptosis in human leukemia U937 cells via formation of reactive oxygen species and opening of mitochondrial permeability transition pores. Int. J. Cancer. 2002;20(99):879–890. doi: 10.1002/ijc.10414. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y.H., Xu K.P., Zhou Y.J. A new flavonol from Sophora tonkinensis. J. Asian Nat. Prod. Res. 2007;9(1):45–48. doi: 10.1080/10286020500289634. [DOI] [PubMed] [Google Scholar]
- 5.Sasajima M., Nakane S., Saziki R. Studies on the anti-ulcer effects of isoprenyl flavonoids (1). The anti-ulcer effects of isoprenyl chalcone extracted from Sophora subprostrata. Yakurigaku Zasshi. 1978;74(8):897–905. doi: 10.1254/fpj.74.897. [DOI] [PubMed] [Google Scholar]
- 6.Luo G.Y., Yang Y., Zhou M. Novel 2-arylbenzofuran dimers and polyisoprenylated flavanones from Sophora tonkinensis. Fitoterapia. 2014;99:21–27. doi: 10.1016/j.fitote.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Pan Q.M., Li Y.H., Hua J., Huang F.P., Wang H.S., Liang D. Antiviral matrine-type alkaloids from the rhizomes of Sophora tonkinensis. J. Nat. Prod. 2015;78(7):1683–1688. doi: 10.1021/acs.jnatprod.5b00325. [DOI] [PubMed] [Google Scholar]
- 8.Ding P.L., Huang H., Zhou P., Chen D.F. Quinolizidine alkaloids with anti-HBV activity from Sophora tonkinensis. Planta Med. 2006;72(9):854–856. doi: 10.1055/s-2006-946639. [DOI] [PubMed] [Google Scholar]
- 9.Akao T., Hayashi T., Kobashi K. Intestinal bacterial hydrolysis is indispensable to absorption of 18 beta-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J. Pharm. Pharmacol. 1994;46:135–137. doi: 10.1111/j.2042-7158.1994.tb03756.x. [DOI] [PubMed] [Google Scholar]
- 10.Zuo F., Zhou Z.M., Zhang Q., Mao D., Xiong Y.L., Wang Y.L., Yan M.Z., Liu M.L. Pharmacokinetic study on the multi-constituents of Huangqin-Tang decoction in rats. Biol. Pharm. Bull. 2003;26:911–919. doi: 10.1248/bpb.26.911. [DOI] [PubMed] [Google Scholar]
- 11.Trinh H.T., Jang S.Y., Han M.J., Kawk H.Y., Beak N.I., Kim D.H. Metabolism of wogonoside by human fecal microflora and its anti-pruritic effect. Biomol. Ther. (Seoul) 2009;17:211–216. [Google Scholar]
- 12.Morgan B.P., Harris C.L. Complement therapeutics; history and current progress. Mol. Immunol. 2003;40:159–170. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 13.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y.Y., Zhang Y.Y., Ou Y.Y., Lu X.X., Pan L.Y., Li H. Houttuyniacordata Thunb. Polysaccharides ameliorates lipopolysaccharide-induced acute lung injury in mice. J. Ethnopharmacol. 2015;173:81–90. doi: 10.1016/j.jep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y., Lu Y., Zhang Y.Y., Chen D.F. Anti-complementary constituents of Houttuynia cordata and their targets in complement activation cascade. Nat. Prod. Res. 2014;28:407–410. doi: 10.1080/14786419.2013.869693. [DOI] [PubMed] [Google Scholar]
- 16.Zhi H.J., Zhu H.Y., Zhang Y.Y., Lu Y., Li H., Chen D.F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine. 2019;57:105–116. doi: 10.1016/j.phymed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Xing S.H., Wang M., Peng Y., Chen D.F., Li X.B. Simulated gastrointestinal tract metabolism and pharmacological activities of water extract of Scutellaria baicalensis roots. J. Ethnopharmacol. 2014;152(1):183–189. doi: 10.1016/j.jep.2013.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabat E.A., Mayer M.M. Springfield: Charles C. Thomas Publisher; 1964. Complement and Complement Fixation in Experimental Immunology; pp. 133–240. [Google Scholar]
- 19.Klerx J.P., Beukelman C.J., van Dijk H., Willers J.M. Microassay for colorimetric estimation of complement activity in Guinea pig, human and mouse serum. J. Immunol. Methods. 1983;63:215–220. doi: 10.1016/0022-1759(83)90425-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Zhang P., Cheng Y. Structural characterization of isoprenylated flavonoids from Kushen by electrospray ionization multistage tandem mass spectrometry. J. Mass Spectrom. 2008;43(10):1421–1431. doi: 10.1002/jms.1423. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q.H., Li X.Z., Han L., Li P.Y., Lu D. Determination of sophorabioside in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. J. Chromatogr. Sci. 2018;56(2):154–159. doi: 10.1093/chromsci/bmx097. [DOI] [PubMed] [Google Scholar]
- 22.Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001;12(6):707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Zhang P., Cheng Y. Structural characterization of isoprenylated flavonoids from Kushen by electrospray ionization multistage tandem mass spectrometry. J. Mass Spectrom. 2008;43(10):1421–1431. doi: 10.1002/jms.1423. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu M., Tomimori T., Hatayama K., Makiguchi Y., Mikuriya N. Studies on the constituents of Sophora species. II. Constituents of Sophora subprostrata CHUN et T. CHEN. (2). Isolation and structure of new flavonoids, sophoradochromene and sophoranochromene. Chem. Pharm Bull. (Tokyo) 1970;18(4):741–745. doi: 10.1248/cpb.18.741. [DOI] [PubMed] [Google Scholar]
- 25.Ye G., Zhu H.Y., Li Z.X., Ma C.H., Fan M.S., Sun Z.L., Huang C.G. LC-MS characterization of efficacy substances in serum of experimental animals treated with Sophora flavescens extracts. Biomed. Chromatogr. 2007;21(6):655–660. doi: 10.1002/bmc.805. [DOI] [PubMed] [Google Scholar]
- 26.Kyogoku K., Hatayama K., Suzuki K., Yokomori S., Maejima K., Komatsu M. Studies on the Constituents of Guang-Dou-Gen (the Root ofSophora subprostrata CHUN et T. CHEN). Isolation of Two New Flavanones. Chem. Pharm Bull. (Tokyo) 1973;21(8):1777–1782. [Google Scholar]
- 27.Zhi H.J., Jin X., Yan H., Zhu H.Li, Zhang Y.Y., Lu Y., Chen D.F. Exploring the effective materials of flavonoids-enriched extract from Scutellaria baicalensis roots based on the metabolic activation in influenza A virus induced acute lung injury. J Pharmaceut Biomed. 2020;177:876–888. doi: 10.1016/j.jpba.2019.112876. [DOI] [PubMed] [Google Scholar]
- 28.Ding P.L., Hou A.J., Chen D.F. Three new isoprenylated flavonoids from the roots of Sophora flavescens. J. Asian Nat. Prod. Res. 2015;7(3):237–243. doi: 10.1080/10286020410001687554. [DOI] [PubMed] [Google Scholar]
- 29.The State Pharmacopoeia Commission of the People’s Republic of China . vol. 1. China Medical Science Press; Beijing: 2010. (Pharmacopoeia of the People’S Republic of China). P. 188. [Google Scholar]
- 30.Krishna P.M., Rao K.N.V., Sandhya S., David B. A review on phytochemical, ethnomedical and pharmacological studies on genus Sophora. Fabaceae. Rev Bras Farmacogn. 2012;22(5):1145–1154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.