Abstract
Background
It is unclear whether multiple respiratory viral infections are associated with more severe bronchiolitis requiring pediatric intensive care unit (PICU) admission. We aimed to identify the association between multiple respiratory viral infections and PICU admission among infants with bronchiolitis.
Methods
We performed a 1:1 case-control study enrolling previously healthy full-term infants (≤12 months) with bronchiolitis admitted to the PICU as cases and those to the general pediatric ward as controls from 2015 to 2017. Multiplex polymerase chain reaction (PCR) was used for detection of the respiratory viruses. We summarized the characteristics of infants admitted to the PICU and the general pediatric unit. Multivariable logistic regression analysis was used to fit the association between multiple respiratory viral infections (≥2 strains) and PICU admission.
Results
A total of 135 infants admitted to the PICU were compared with 135 randomly selected control infants admitted to the general pediatric unit. The PICU patients were younger (median: 2.2 months, interquartile range: 1.3–4.2) than the general ward patients (median: 3.2 months, interquartile range: 1.6–6.4). Respiratory syncytial virus (74.1%), rhinovirus (28.9%), and coronavirus (5.9%) were the most common viruses for bronchiolitis requiring PICU admission. Patients with bronchiolitis admitted to the PICU tended to have multiple viral infections compared with patients on the general ward (23.0% vs. 10.4%, P < 0.001). In the multivariable logistic regression analysis, bronchiolitis with multiple viral infections was associated with higher odds of PICU admission (adjusted odds ratio: 2.56, 95% confidence interval: 1.17–5.57, P = 0.02).
Conclusion
Infants with multiviral bronchiolitis have higher odds of PICU admission compared with those with a single or nondetectable viral infection.
Keywords: Bronchiolitis, Infants, Pediatric intensive care, Multiple respiratory viral
1. Introduction
Bronchiolitis is an acute viral respiratory infection that is characterized by bronchiolar obstruction with edema, mucus, and cellular debris in children under 2 years of age. Affected children typically have fever, cough, and respiratory distress (e.g., retraction, wheezing) [1]. Bronchiolitis is an important issue as it is one of the leading causes of hospitalization among children. The total annual costs for bronchiolitis-related hospitalizations in the United States were $543 million [2]. Approximately 2–3% of affected children require hospitalization for a higher level of care, leading to 149,000 hospitalizations annually [2]. The majority of children with bronchiolitis have mild-to-moderate illness, but 2–6% of hospitalized children require pediatric intensive care unit (PICU) admission, and 2–3% of the hospitalized children need high-flow oxygenation or mechanical ventilation [3].
Established risk factors for hospital admission or PICU admission include prematurity, younger age, the presence of a comorbidity, and environmental factors. Respiratory syncytial virus (RSV) is most frequently associated with bronchiolitis, accounting for 43–74% of all cases [4]. The recent introduction and widespread use of molecular-based methods (real-time multiplex polymerase chain reaction [PCR]) has helped identify an increased number of concomitant viral infections (e.g., metapneumovirus) in addition to RSV in children with acute bronchiolitis. Other viruses frequently found in children with bronchiolitis include rhinovirus, coronavirus, human metapneumovirus, parainfluenza virus, and adenovirus [5], [6]. Concomitant viral infection in hospitalized children with bronchiolitis ranges from 10% to 40% [7], [8], [9], [10], [11]. However, it remains unanswered whether multiple concomitant respiratory viral infections are associated with more severe bronchiolitis. Prior studies were limited for only providing descriptive statistics without accounting for confounders or for having inadequate sample size [10], [11], [12]. It is important to know whether multiple respiratory viral infections are linked to worse respiratory outcomes, since early intensive care given to this vulnerable population might decrease the need for rapid response on the pediatric ward and potentially adverse outcomes. To address these issues, we conducted a case-control study and performed a multivariable regression analysis to determine the association between severe bronchiolitis resulting in PICU admission and the number of viral co-infections among previously healthy infants hospitalized for acute bronchiolitis, while accounting for confounders.
2. Material and methods
2.1. Study design
This study was approved by the Driscoll Children's Hospital Institutional Review Board. This was a 1:1 case-control study of infants aged 12 months or younger who were admitted to our 18-bed tertiary care pediatric intensive care unit (PICU). The study sample was chosen based on the ICD code for bronchiolitis during the study period. Infants with bronchiolitis admitted to the PICU were identified as cases and were compared with infants with bronchiolitis admitted to the general pediatric unit as controls. As cases, we included all patients with bronchiolitis admitted to the PICU from whom a nasopharyngeal swab (NPS) sample had been collected for real-time multiplex PCR at our institution from January 2015 to December 2017. PCR analysis was performed based on the physician's decision regarding the need for additional testing that might change patient management. As controls, the same number of neonates (<28 days; n = 20) and infants (n = 135) were randomly selected among the 890 patients (age ≤ 1 year) with bronchiolitis admitted to the general pediatric unit, and for whom PCR was performed during the same study period, using a random sampling method. Sensitivity analysis was conducted to determine whether there was a significant difference between patients with bronchiolitis admitted to the general pediatric unit for whom PCR was performed and those for whom PCR was not performed. We retrospectively reviewed the electronic medical records (EMR) of eligible patients and documented the demographic, clinical, laboratory, imaging, and outcome data. Patients who were premature or had significant underlying medical illness (e.g., tracheostomy, baseline oxygen requirement, cyanotic heart disease, chromosomal disorders, neurologic disorders) were excluded from the study.
2.2. Exposure of interest: respiratory viral infection
The real-time multiplex PCR used in our institution is the FilmArray Respiratory Panel 2 (RP2) by BioFire that allows for rapid detection (around 45 min) of 22 viral and atypical bacterial pathogens directly from NPS samples. The detectable pathogens include adenovirus, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43, human metapneumovirus, human rhinovirus/enterovirus, influenza virus A, influenza virus A H1, influenza virus A H1-2009, influenza virus A H3, influenza virus B, parainfluenza virus 1, parainfluenza virus 2, parainfluenza virus 3, parainfluenza virus 4, respiratory syncytial virus, Bordetell a pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae, Middle East respiratory syndrome – coronavirus, and Bordetella parapertussis. FilmArray RP2 is a reliable and accurate assay that has an overall percentage of agreement of 99.2% with the gold standard [13]. Infection with multiple viruses is defined as the presence of two or more viral strains.
2.3. Outcome measures
The primary outcome was admission to the PICU, as a surrogate outcome for the severity of bronchiolitis. The criteria for admission to the PICU in our institution include, but are not limited to, hypoxia with oxygen requirement of 40% or respiratory distress requiring mechanical and nonmechanical ventilation (e.g., high-flow oxygen), hemodynamic instability not responding to initial fluid resuscitation (i.e., 60 mL/kg normal saline bolus), or the clinicians’ discretion. Our secondary outcome was intubation after hospital admission.
2.4. Statistical analysis
Exploratory data analysis was conducted to describe the characteristics of infants with bronchiolitis admitted to the general pediatric ward and the PICU. Further, the type and the number of respiratory viral infections were compared between infants admitted to the PICU and those admitted to the general pediatric ward. Categorical variables are presented as frequency and percentage, and were compared using the Chi2 test. Continuous variables are presented as mean or median and were compared with the t test or Wilcoxon rank-sum test as appropriate, for non-normally distributed continuous variables and normally distributed continuous variables.
To assess the association between the number of respiratory viral infections and primary (i.e., PICU admission) as well as secondary outcomes (i.e., intubation), multivariable logistic regression analyses controlling for potential confounders were conducted. The following factors were adjusted for: age category (neonates, infants), weight, bronchodilator use, feeding type, antibiotic use, systemic steroid use, and heart rate at admission. Covariates were initially determined based on the potential relevance reported in the literature and on their association with the outcome in the bivariate analysis at a significance level of P < 0.1. Statistical analysis was conducted using Stata 12.0 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Patient demographics and clinical characteristics
During the study period, there were 135 infants in the PICU who were compared with 135 control infants randomly selected out of 890 infants admitted to general pediatric ward. Among 135 patients admitted to the PICU, 20 patients (14.8%) were neonates, of whom 61 (45.2%) were female, with a median age of 2.2 months (interquartile range [IQR]: 1.3–4.2 months). By comparison, among 135 patients admitted to the general pediatric ward as controls, 20 patients (14.8%) were neonates, of whom 54 (40.0%) were female, with a median age of 3.2 months (IQR: 1.6–6.4 months). The characteristics between patients with bronchiolitis admitted to the PICU and those admitted to the general pediatric ward are summarized in Table 1 . Compared with patients admitted to the general pediatric ward, patients admitted to the PICU were younger, weighed less, were more likely to have received bronchodilators, antibiotics, feeding tube (NG/ND), and had a longer hospital stay. There was no mortality among the cases or the controls.
Table 1.
Characteristics of bronchiolitis patients with PICU admission and non-PICU admission.
| Non-PICU admission (n = 135) |
PICU admission (n = 135) |
P | |
|---|---|---|---|
| Ageinmonths, median(IQR) | 3.2 (1.6, 6.4) | 2.2 (1.3, 4.2) | 0.015 |
| Neonate, n (%) | 20 (14.8) | 20 (14.8) | 1.00 |
| Female, n (%) | 54 (40.0) | 61 (45.2) | 0.39 |
| Weight(kg), median(IQR) | 7.1 (5.0, 11.5) | 4.9 (3.9, 6.1) | <0.001 |
| WBC(103/μL), median(IQR) | 10.5 (7.7, 13.9) | 9.6 (7.3, 12.6) | 0.31 |
| Hgb(g/dL), median(IQR) | 11.7 (10.8, 12.5) | 11.1 (10.2, 12.0) | 0.047 |
| Bronchodilatoruse, n (%) | 36 (26.7) | 84 (62.2) | <0.001 |
| ICSuse, n (%) | 33 (24.4) | 9 (6.7) | <0.001 |
| Systemicsteroiduse, n (%) | 44 (32.6) | 22 (16.3) | 0.002 |
| HTSuse, n (%) | 115 (85.2) | 125 (92.6) | 0.053 |
| Antibioticuse, n (%) | 37 (27.4) | 84 (62.2) | <0.001 |
| HRathospitaladmission, median(IQR) | 155.0 (137.0, 173.0) | 166.0 (150.0, 180.0) | <0.001 |
| RRathospitaladmission, median(IQR) | 46.0 (40.0, 55.0) | 48.0 (39.0, 61.0) | 0.14 |
| O2 flowathospitaladmission, median(IQR) | 0.0 (0.0, 0.0) | 3.0 (1.0, 8.0) | <0.001 |
| SpO2 athospitaladmission, median(IQR) | 98.0 (97.0, 100.0) | 98.0 (95.0, 100.0) | 0.45 |
| DurationofAbx, median(IQR) | 48.0 (48.0, 72.0) | 48.0 (24.0, 72.0) | 0.15 |
| Feedingmethod, n (%) | |||
| NG/ND | 19 (14.1) | 69 (51.1) | <0.001 |
| PO | 116 (85.9) | 66 (48.9) | |
| Lengthofhospitalstay(h), median(IQR) | 44.0 (30.0, 67.0) | 130.0 (94.0, 214.0) | <0.001 |
IQR: interquartile range; PICU: pediatric intensive care unit; ICS: inhaled corticosteroids; HTS: hypertonic saline; HR: heart rate; RR: respiratory rate; NG: nasogastric; ND: nasoduodenal; PO: per os; h: hour.
3.2. Respiratory viral profiles
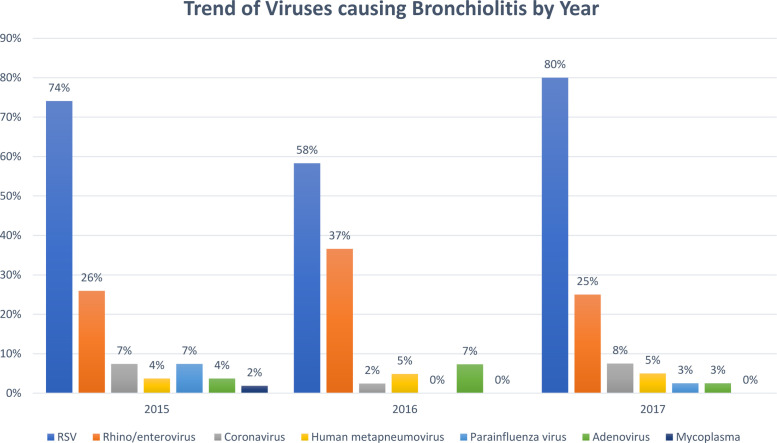
The type and the number of respiratory viruses detected via PCR are summarized in Table 2 . During the study period, 87.75% (781/890) of patients diagnosed with bronchiolitis and admitted to the general ward had PCR testing, while 100% (135/135) of patients diagnosed with bronchiolitis admitted to the PICU had PCR testing. There were no significant differences in age, gender, and length of hospital stay between those admitted to the general ward with and without PCR testing (P > 0.05). All study subjects (135 cases and 135 controls) were tested for viral PCR either prior to admission or during their stay in the hospital. PCR detected one or more viruses in 132 (97.8%) and 100 (74.1%) infants admitted to the PICU and to the general ward, respectively. Infections with a single virus and multiple viruses were found in 101 (74.8%) and 31 (23.0%) of infants admitted to the PICU, respectively. On the other hand, infections with a single virus and multiple viruses were found in 86 (63.7%) and 14 (10.4%) of infants admitted to the general ward, respectively. RSV, rhinovirus, and coronavirus were the top three causes of acute bronchiolitis for both the patients admitted to the general ward and the PICU. Compared with patients with bronchiolitis admitted to the general ward, those admitted to the PICU were more likely to have co-infection of RSV and adenovirus (0% vs. 4.4%, P = 0.013), as well as RSV and mycoplasma (3.7% vs. 11.9%, P = 0.012) (Table 2). Fig. 1 shows the proportion of PICU patients with bronchiolitis caused by each respiratory virus for the period 2015–2017. Compared with the patients admitted to the general ward, those admitted to the PICU were more likely to have multiple viral infections and a high average number of respiratory viral infections (Table 2).
Table 2.
Respiratory viral characteristics and types by PICU admission status.
| Non-PICU admission (n = 135) |
PICU admission (n = 135) |
P | |
|---|---|---|---|
| Multiplevirusinfection, n (%) | |||
| None | 35 (25.9) | 3 (2.2) | <0.001 |
| Single | 86 (63.7) | 101 (74.8) | |
| Multiple | 14 (10.4) | 31 (23.0) | |
| Numbersofvirusinfection, mean(SD) | 0.9 (0.6) | 1.2 (0.5) | <0.001 |
| Typeofrespiratoryvirus/organism | |||
| RSV, n (%) | 79 (58.5) | 100 (74.1) | 0.007 |
| Coronavirus, n (%) | 7 (5.2) | 8 (5.9) | 0.79 |
| Human metapneumovirus, n (%) | 2 (1.5) | 6 (4.4) | 0.15 |
| Parainfluenza virus, n (%) | 8 (5.9) | 5 (3.7) | 0.39 |
| Adenovirus, n (%) | 2 (1.5) | 6 (4.4) | 0.15 |
| Mycoplasma, n (%) | 0 (0.0) | 1 (0.7) | 0.32 |
| Rhinovirus/enterovirus, n (%) | 17 (12.6) | 39 (28.9) | <0.001 |
| Typesofviral/organismco-infection | |||
| RSV + coronavirus, n (%) | 5 (3.7) | 7 (5.2) | 0.55 |
| RSV + human metapneumovirus, n (%) | 0 (0.0) | 1 (0.7) | 0.32 |
| RSV + parainfluenza virus, n (%) | 3 (2.2) | 1 (0.7) | 0.31 |
| RSV + adenovirus, n (%) | 0 (0.0) | 6 (4.4) | 0.013 |
| RSV + mycoplasma, n (%) | 5 (3.7) | 16 (11.9) | 0.012 |
| Coronavirus + adenovirus, n (%) | 1 (0.7) | 1 (0.7) | 1.00 |
| Parainfluenza virus + mycoplasma, n (%) | 0 (0.0) | 1 (0.7) | 0.32 |
| Parainfluenza virus + rhinovirus/enterovirus, n (%) | 2 (1.5) | 2 (1.5) | 1.00 |
PICU: pediatric intensive care unit; SD: standard deviation; RSV: respiratory syncytial virus. There were no detectable co-infection for RSV + rhinovirus/enterovirus, coronavirus + human metapneumovirus, coronavirus + parainfluenza virus, coronavirus + mycoplasma, coronavirus + rhinovirus/enterovirus, human metapneumovirus + parainfluenza virus, human metapneumovirus + adenovirus, human metapneumovirus + mycoplasma, human metapneumovirus + rhinovirus/enterovirus, parainfluenza virus + adenovirus, adenovirus + mycoplasma, adenovirus + rhinovirus/enterovirus, human metapneumovirus + rhinovirus/enterovirus.
Fig. 1.
Trend of respiratory virus/organism causing bronchiolitis associated with PICU admission for the period 2015–2017. PICU: pediatric intensive care unit.
3.3. Association between multiple viral infections and PICU admission as well as endotracheal intubation
In multivariable logistic regression models adjusted for age category, gender, weight, bronchodilator use, feeding type, antibiotic use, systemic steroid use, and heart rate at hospital admission, bronchiolitis with multiple viral infections (≥2 strains) was associated with higher odds of PICU admission (adjusted odds ratio [OR]: 2.42, 95% CI: 1.14–5.27, P = 0.03). Further, the odds of having bronchiolitis associated with PICU admission increased with the number of viral infections at admission (adjusted OR: 2.86, 95% CI: 1.28–6.42, P = 0.01). For the secondary outcome, multiple respiratory viral infections were not associated with increased odds of endotracheal intubation in the multivariable logistic regression (adjusted OR: 0.82, 95% CI: 0.19–3.44, P = 0.78) (Table 3 ).
Table 3.
Association between multiple viral infections and outcome of hospitalized bronchiolitis patientsa.
| PICU admission |
||||
|---|---|---|---|---|
| Unadjusted model |
Adjusted model |
|||
| Predictor | OR (95% CI) | P | OR (95% CI) | P |
| Number of viral infections | 3.57 (2.13–5.97) | <0.01 | 2.42 (1.14–5.27) | 0.03 |
| Multiple viral infectionsb | 3.89 (2.28–6.64) | <0.01 | 2.86 (1.28–6.42) | 0.01 |
PICU: pediatric intensive care unit; OR: odds ratio; CI: confidence interval.
Variables adjusted in multivariable logistic/linear regression: age categories, weight, bronchodilator use, feeding type, antibiotic use, systemic steroid use, heart rate at admission.
Multiple viral infection: ≥2 strains.
4. Discussion
This case-control study is important from epidemiological and clinical perspectives. It summarizes the prevalence of multiple respiratory viral infections from a single hospital cohort for three consecutive years. Furthermore, it confirms and quantifies the independent association between multiple viral infections and severe bronchiolitis leading to PICU admission using a multivariable logistic regression model that controls for potential confounders. Our findings are crucial as they could help clinicians readily identify a vulnerable population more likely to develop severe bronchiolitis who may require PICU admission. Close monitoring and early intervention could be implemented for this population to decrease morbidity and mortality associated with bronchiolitis.
RSV is the most commonly detected virus both for children with bronchiolitis admitted to the PICU and for those admitted to the general pediatric ward. The RSV detection rates of 74% and 59% for cases and controls, respectively, were compatible with historical numbers (60–80%) reported in various studies [14], [15], [16]. Similarly, rhinovirus was the second most common virus found in 27% and 12% of the children admitted to the PICU and the general pediatric ward, respectively, while the detection rate reported in the literature was 20–40% [10], [12], [17]. Interestingly, different from the results reported in previous studies, the third most common virus detected among children admitted to the PICU in this study varies according to the year. Potential reasons could be the yearly and geographical variation in the viral prevalence. Secondly, different study populations might partially explain the variation, as the majority of patients in southern Texas are of Hispanic origin. Similar observations were also reported in studies from France and Taiwan, where only RSV was consistently found to be the most common of all viruses [7], [8].
Consistent with the prevalence reported in previous studies [18], our findings revealed that infection with multiple respiratory viruses accounts for 10% and 22% of children admitted to the general ward and the PICU, respectively. Further, we found a significantly higher percentage of multiple viral infections among the PICU cohort. Additionally, the results of multivariable analyses suggest that multiple respiratory viral infections were independently associated with higher odds of severe bronchiolitis leading to PICU admission, while accounting for confounders. This finding is different from the results reported by Nascimento et al., who suggested no significant association between multiple viral infections and PICU admission for children admitted to hospital with bronchiolitis [12]. Several reasons might explain the difference. First, the study populations might be different. Patient selection bias might exist in their study considering that the study was conducted in a neighborhood of residents of high socioeconomic status. Secondly, their relatively small sample size (n = 77) might not afford adequate statistical power to determine the significant association between multiple viral infections and PICU admission. In fact, our findings from multivariable analyses agree with the results of a retrospective study by Richard et al. [8], in which multiple respiratory viral infections were found to associated with higher odds of PICU admission (adjusted OR: 2.7, 95% CI: 1.2–6.2, P = 0.02) on multivariable analysis. In addition to having higher odds of PICU admission, bronchiolitis with multiple viral infections was found to be associated with prolonged PICU stay in a British retrospective cohort study enrolling children under 2 years of age admitted to the PICU for bronchiolitis [17]. The intriguing finding that infection with multiple respiratory viruses is associated with more severe bronchiolitis is biologically plausible via several proposed mechanisms, including virus-derived disruption of the epithelium and modification of the host immune system [19]. Taken together, infection with multiple respiratory viruses is associated with more severe bronchiolitis and prolonged PICU stay.
Real-time multiplex PCR that detects the presence of nucleic acid from several viruses or bacteria from nasopharyngeal secretion in a single test allows for timely recognition of pathogens with high sensitivity and specificity [20]. While the rapid recognition of pathogens may help clinicians make decisions, one must be aware that the presence of viral nucleic acid based on PCR does not mean active viral infection, as it could result from subclinical viral infection or prior viral infection since the viral nucleic acid remnant may last up to 4 weeks after infection [21], [22]. Therefore, the results of multiplex PCR must be interpreted together with the patients’ clinical presentation. However, there is also evidence suggesting that subclinical viral infection leads to alteration of the host-microbe composition of the respiratory tract, which might be linked to subsequent dysregulation in host immune function associated with development of asthma or against bacterial infection [23], [24]. The impact of altered microbiota in the respiratory tract after viral infections awaits future studies.
There is no clear evidence that the use of real-time multiplex PCR could shorten the average length of hospital stay or the duration of antibiotic use, but PCR might lead to reduced exposure to chest X-ray [25], [26]. Furthermore, there is no consensus on when respiratory PCR should be performed for pediatric patients. In general, respiratory PCR is recommended for neonates, immunosuppressed patients, or patients with chronic cardiopulmonary illness because a positive result might consequently lead to antiviral/antimicrobial treatments or clinical interventions. Based on the findings of this study, we also recommend respiratory PCR for patients with a more severe presentation of bronchiolitis as it may help clinicians decide on the patient's condition and the level of care needed. This is because patients with a more severe presentation of bronchiolitis with unremarkable chest X-rays but positive PCR results for co-infection with multiple viruses might benefit from closer monitoring as they are more likely to experience a more severe course of bronchiolitis requiring PICU admission.
The results of this study should be interpreted in the context of its strength and limitations. Our study reconfirms the independent association between multiple respiratory viral infections and severe bronchiolitis requiring PICU admission. However, this study has several limitations. First, using PICU admission as a surrogate outcome for severe bronchiolitis might introduce bias. It might not reflect directly the respiratory burden imposed by the multiple viral infections as accurately as respiratory rate or oxygen saturation level. Nevertheless, using PICU admission as a surrogate outcome takes account of the cumulative impact of respiratory viral infections, not only on the respiratory system but also on other organ systems, and of the interventions already administered. Therefore, it is reasonable to use of PICU admission as a surrogate outcome. Second, as with other retrospective studies, our results might be confounded by non-measured variables, such as exposure to cigarettes, duration of breastfeeding, number of siblings, socioeconomic status, personal or family history of atopy, or undiagnosed asthma. As our study subjects were previously healthy full-term infants, the extent of the confounding effect would be to a lesser degree. Further, adjustment for the use of steroids, inhaled corticosteroids, and antibiotics in the multivariable analysis helps control these confounding effects. Third, our study population is relatively selective as we only enrolled previously healthy full-term infants hospitalized for bronchiolitis who had respiratory viral panel testing via PCR. Thus, the results might not be generalizable to other populations (e.g., premature infants). Furthermore, the decision regarding admission to the PICU might not be entirely objective, as it was at the discretion of clinicians who could not be entirely blinded to the patients’ clinical status. However, at our institution there were established criteria that prevent unjustifiable admission to the PICU. Fourth, as with other clinical studies using ICD codes to ascertain diseases from administrative databases, our study is also at risk of misclassification bias as the bronchiolitis might be erroneously diagnosed or missed. Fifth, one must be aware that positive PCR results do not necessarily mean active infection, as there could be viral residual shedding from previous infections. PCR should always be interpreted along with the patients’ clinical presentation.
5. Conclusion
In summary, we identified the independent association between infection with multiple respiratory viruses and severe bronchiolitis requiring PICU admission. Infants with bronchiolitis due to multiple viruses may require PICU care more frequently than infants with bronchiolitis due to a single or non-detectable viral infection. Bronchiolitis caused by multiple viruses is not associated with a higher need for invasive mechanical ventilation. Establishing this association may help clinicians with early identification of high-risk patients who might benefit from closer monitoring in the PICU and early institution of escalated care.
Ethical approval
This study was approved by the Driscoll Children's Hospital Institutional Review Board.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
We thank the staff at Driscoll Children's Hospital for the contribution of technical support with the data.
References
- 1.Florin T.A., Plint A.C., Zorc J.J. Viral bronchiolitis. Lancet. 2017;389:211–224. doi: 10.1016/S0140-6736(16)30951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier A.J., Mansbach J.M., Camargo C.A., Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa K., Tsugawa Y., Brown D.F. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansbach J.M., Piedra P.A., Teach S.J. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166:700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 6.Shay D.K., Holman R.C., Newman R.D. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y.W., Huang Y.C., Ho T.H. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Infect. 2014;47:116–121. doi: 10.1016/j.jmii.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richard N., Komurian-Pradel F., Javouhey E. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr Infect Dis J. 2008;27:213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 9.Marguet C., Lubrano M., Gueudin M. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4:e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand H.K., de Groot R., Galama J.M. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol. 2012;47:393–400. doi: 10.1002/ppul.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadopoulos N.G., Moustaki M., Tsolia M. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento M.S., Souza A.V., Ferreira A.V. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics (Sao Paulo) 2010;65:1133–1137. doi: 10.1590/S1807-59322010001100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leber A.L., Everhart K., Daly J.A. Multicenter evaluation of biofire filmarray respiratory panel 2 for detection of viruses and bacteria in nasopharyngeal Swab samples. J Clin Microbiol. 2018:56. doi: 10.1128/JCM.01945-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jartti T., Lehtinen P., Vuorinen T. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28:311–317. doi: 10.1097/INF.0b013e31818ee0c1. [DOI] [PubMed] [Google Scholar]
- 15.Oymar K., Skjerven H.O., Mikalsen I.B. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014;22:23. doi: 10.1186/1757-7241-22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson R.F. Impact of respiratory syncytial virus in the United States. Am J Health Syst Pharm. 2008;65:S3–S6. doi: 10.2146/ajhp080438. [DOI] [PubMed] [Google Scholar]
- 17.Ghazaly M., Nadel S. Characteristics of children admitted to intensive care with acute bronchiolitis. Eur J Pediatr. 2018;177:913–920. doi: 10.1007/s00431-018-3138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robledo-Aceves M., Moreno-Peregrina M.J., Velarde-Rivera F. Risk factors for severe bronchiolitis caused by respiratory virus infections among Mexican children in an emergency department. Medicine. 2018;97:e0057. doi: 10.1097/MD.0000000000010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch A.A., Biesbroek G., Trzcinski K. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause J.C., Panning M., Hengel H. The role of multiplex PCR in respiratory tract infections in children. Dtsch Arztebl Int. 2014;111:639–645. doi: 10.3238/arztebl.2014.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffelholz M.J., Trujillo R., Pyles R.B. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134:1144–1150. doi: 10.1542/peds.2014-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munywoki P.K., Koech D.C., Agoti C.N. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect. 2015;143:804–812. doi: 10.1017/S0950268814001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanada S., Pirzadeh M., Carver K.Y. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu P., Hartert T.V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wishaupt J.O., Russcher A., Smeets L.C. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113–e11120. doi: 10.1542/peds.2010-2779. [DOI] [PubMed] [Google Scholar]
- 26.Doan Q., Enarson P., Kissoon N. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD006452.pub3. [CD006452] [DOI] [PubMed] [Google Scholar]