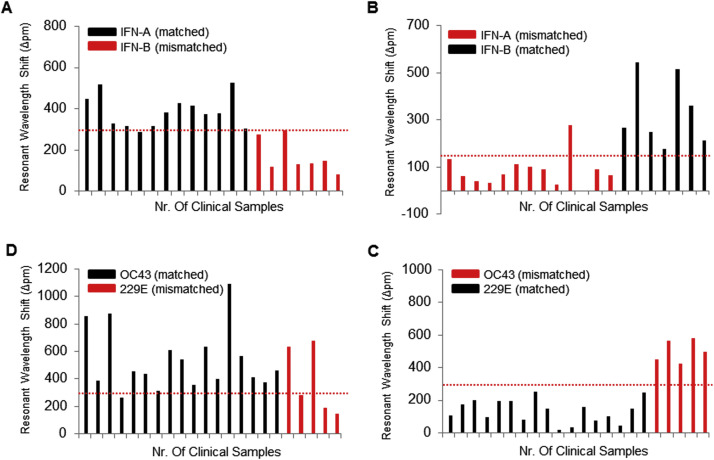
Fig. 5.
Cross-reactivity testing of the iROAD assay in clinical samples. (A) Analysis of 20 clinical nasopharyngeal samples from 13 IFN-A patients (as targets, black) and 7 IFN-B patients (as non-targets, red) with IFN-A primer in 30 min (B) Analysis of 20 clinical nasopharyngeal samples from 13 IFN-A patients (as non-targets, red) and 7 IFN-B patients (as targets, black) with IFN-B primer in 30 min (C) Analysis of 22 clinical nasopharyngeal samples from 17 HCoV-OC43 patients (as targets, black) and 5 HCoV-229E patients (as non-targets, red) with HCoV-OC43 primer in 30 min (D) Analysis of 22 clinical nasopharyngeal samples from 17 HCoV-OC43 patients (as non-targets, red) and 5 HCoV-229E patients (as targets, black) with HCoV-229E primer in 30 min. The red dots represent the cut off (criterion) for reporting a sample as virus (positive/negative) detected. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)