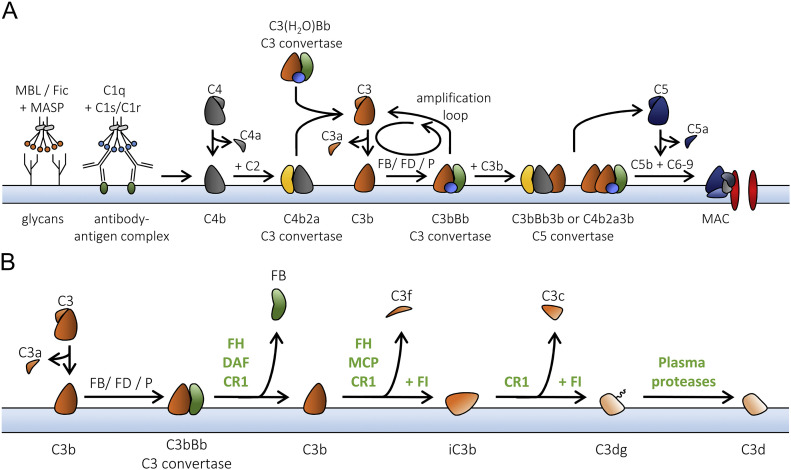
Fig 1.
A, The complement system. The complement system is initiated by the binding of recognition molecules from the lectin or the classical pathway (LP and CP). In the LP, MBL or ficolins (Fic) are complexed with MASP proteins and recognize glycan moieties on a pathogenic surface. The C1 complex, composed of C1q and 2 molecules of C1r and C1s each, recognizes antibody-antigen complexes. After activation of the MASP proteins or C1r/C1s, C4 is cleaved by C1s into C4b, which deposits on the surface via its thioester. The subsequent cleavage of C2 by C1s allows for the formation of the C4b2a C3 convertase—able to cleave C3 into C3b. Via the AP, a C3 convertase, can also form in the fluid phase, composed of C3(H2O) and Bb (the large product upon FB cleavage by FD), or as C3bBb deposited on the surface. Both these AP convertases are stabilized with properdin (P). The alternative pathway functions as an amplification loop, increasing the deposition of C3b. When the number of deposited C3b molecules increases, C5 becomes the preferred substrate for the convertases, initiating the terminal pathway. C5b, together with C6, C7, C8, and several molecules of C9, forms a lytic pore known as the membrane-attack complex (MAC).
B, The breakdown of deposited C3b. C3b that is attached to a surface can be regulated via multiple mechanisms. FH, DAF (CD55), and complement receptor 1 (CR1) are able to compete with FB to prevent formation or accelerate the decay of the AP C3 convertase. In addition, FH, MCP (CD46), and CR1 function as cofactors for the serine protease FI to cleave C3b into its inactive form, iC3b, releasing the C3f fragment. CR1 also aids FI to cleave iC3b into C3dg. Other plasma proteases will cleave off the last C3g fragment, leaving the last antigenic fragment, C3d, attached to the surface.