Figure 5.
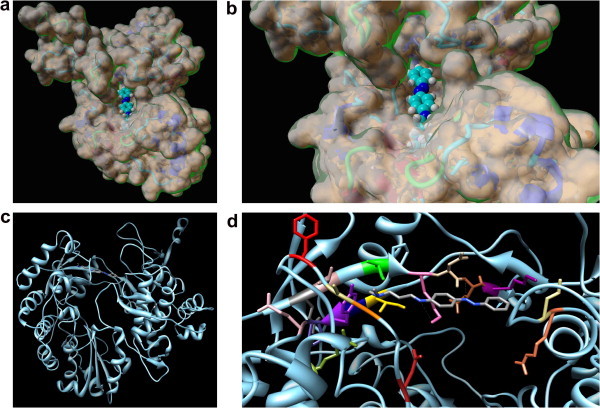
Overall (a, top left) and detailed (b, top right) space filling representation of the BVDV RdRp molecular surface and compound 1 docked into the protein putative binding site. The inhibitor is in CPK representation, with carbons in cyan, nitrogens in blue, and hydrogens in white. (c, bottom left) Ribbon diagram of BVDV RdRp/1 complex structure as resulting from the applied docking/MD procedure. The protein is colored light blue. The inhibitor 1 is represented as a stick model with carbons in gray and nitrogens in blue. (d, bottom right) Details of compound 1 (in a stick representation) in the binding pocket in the enzyme fingers domain. Color scheme as above. The side chains of all residues that form the primary binding pocket interacting with compound 1 are shown as stick models, and the atom color-coding is as follows: N217, firebrick; A221, orange; A222, dark kaki; F224, red; E258, dim gray; T259, rosy brown; I261, green; K263, hot pink; N264, tan; E265, sienna; K266, dark magenta; I287, gold; Q288, navy blue; Y289, purple; P290, dark slate blue; E291, pink; R295, olive drab; R529, coral; L530, kaki. Hydrogen bonds are highlighted as light gray broken lines. Hydrogen atoms, counterions, and water molecules are omitted for clarity.
