Figure 3.
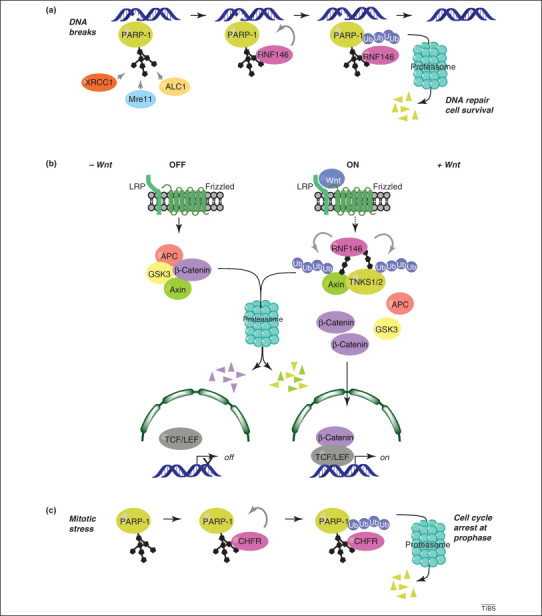
poly(ADP-ribose) (PAR)-dependent poly-ubiquitylation of proteins to promote their degradation by the proteasome. (a) PAR-dependent activation of RNF146 by poly(ADP-ribose) polymerase (PARP)-1 in the DNA damage response. PARP-1 detects DNA breaks, is activated, and auto- or heteromodifies acceptor proteins. PAR produced at DNA damage site has a signaling role and directly recruits factors involved in regulation of chromatin structure and DNA repair [for example amplified in liver cancer 1 (ALC1), X-ray repair cross-complementing gene 1 (XRCC1) or meiotic recombination 11 (Mre11), see Box 1]. The ubiquitin E3 ligase RNF146 is activated upon binding to poly(ADP-ribosyl)ated (PARylated) PARP-1 and poly-ubiquitylates PARP-1. Ubiquitylated PARP-1 is subsequently targeted to the proteasome for degradation. The timely and orchestrated poly(ADP-ribosyl)ation and ubiquitylation of PARP-1 regulates DNA repair and favors cell survival. (b) PAR-dependent activation of RNF146 by tankyrases during Wnt signaling. In the absence of Wnt, the multiprotein β-catenin destruction complex, which contains adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3) and axin triggers the proteasome-dependent degradation of β-catenin. The Wnt signaling cascade is activated by binding of Wnt to its receptor, the low-density lipoprotein receptor-related protein LRP/Frizzled. Tankyrase 1 and 2 (TNKS1/2) PARylate themselves and axin. How TNKS1/2 are activated during Wnt signaling is unknown, and it remains to be determined by which mechanism the activation signal is transmitted from Wnt receptor to TNKS1/2 (illustrated by a dashed arrow between the Wnt receptor and TNKS1/2/RNF146 complex). The PAR activates the ubiquitin E3 ligase RNF146, leading to TNKS1/2 and axin poly-ubiquitylation and subsequent degradation by the proteasome. The multiprotein β-catenin destruction complex is destabilized by the absence of axin, leading to β-catenin accumulation and translocation into the nucleus, where it coactivates the T-cell factor/lymphoid enhancer (TCF/LEF) transcription factor, to promote transcription of Wnt-dependent genes. (c) PAR-dependent activation of CHFR [checkpoint with forkhead (FHA)-associated and really interesting new gene (RING) finger domains] by PARP-1 during mitotic stress. In response to mitotic stress caused by drugs that affect microtubules, such as nocodazole or docetaxel, PARP-1 is activated by an as-yet-unknown process, leading to its auto-PARylation. This results in the PAR-dependent activation of the ubiquitin E3 ligase CHFR and subsequent poly-ubiquitylation of PARP-1 and degradation by the proteasome. Removal of PARylated PARP-1 is necessary to promote cell cycle arrest at prophase.
