Figure 2.
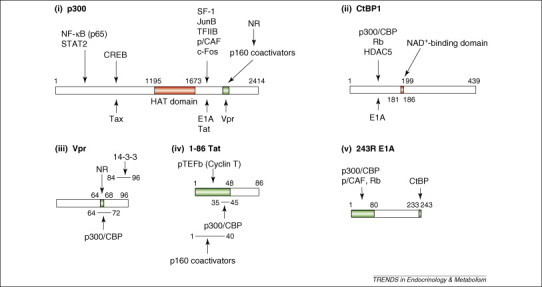
Linearized diagrams of (i) p300, (ii) CtBP1, (iii) Vpr, (iv) Tat, and (v) E1A and their mutual-interaction domains. Numerous transcription factors, transcriptional regulators and viral molecules bind the transcriptional coactivator p300 or its homolog CBP. Binding sites of p160 NR coactivators and Vpr overlap, and both bind NRs and p300 or CBP. Thus, Vpr mimics the host p160 NR coactivators and enhances NR transcriptional activity. p300/CBP facilitates attraction of transcription factors, cofactors and general transcription complexes by loosening the histone–DNA interaction through acetylation of histone tails by its HAT domain. The N-terminal portion of CtBP1 interacts physically with HDAC5 and Rb, which repress transcription. CtBP1 regulates interaction with its binding partners by sensing NADH levels through its NAD+-binding domain. E1A binds to the C-terminal portion of p300 and associates physically with the N-terminal portion of CtBP1 through its C-terminal end. The HAT domain of p300 and the NAD+-binding domain of CtBP1 are indicated in red. This figure is based on Refs 16, 33, 45. Abbreviations: CREB, CRE-binding protein; HAT, histone acetyltransferase; HDAC5, histone deacetylases 5; NF-κB, nuclear factor-κB; NAD, nicotinamide adenine dinucleotide; NR, nuclear hormone receptor; p/CAF, p300/CBP-associating factor; pTEFb, positive-acting transcription elongation factor b; Rb, retinoblastoma protein; SF-1, steroidogenic factor-1; STAT2, signal transducer and activator of transcription 2; TFIIB, transcription factor IIB.
