Abstract
Objectives
To determine efficacy and safety of withholding antimicrobials in children with cancer, fever and neutropenia (FN) with a demonstrated respiratory viral infection.
Methods
Prospective, multicentre, randomized study in children presenting with FN at five hospitals in Santiago, Chile, evaluated at admission for diagnosis of bacterial and viral pathogens including PCR-microarray for 17 respiratory viruses. Children positive for a respiratory virus, negative for a bacterial pathogen and with a favourable evolution after 48 h of antimicrobial therapy were randomized to either maintain or withhold antimicrobials. Primary endpoint was percentage of episodes with uneventful resolution. Secondary endpoints were days of fever/hospitalization, bacterial infection, sepsis, admission to paediatric intensive care unit (PICU) and death.
Results
A total of 319 of 951 children with FN episodes recruited between July 2012 and December 2015 had a respiratory virus as a unique identified microorganism, of which 176 were randomized, 92 to maintain antimicrobials and 84 to withdraw. Median duration of antimicrobial use was 7 days (range 7–9 days) versus 3 days (range 3–4 days), with similar frequency of uneventful resolution (89/92 (97%) and 80/84 (95%), respectively, not significant; OR 1.48; 95% CI 0.32–6.83, p 0.61), and similar number of days of fever (2 versus 1), days of hospitalization (6 versus 6) and bacterial infections throughout the episode (2%–1%), with one case of sepsis requiring admission to PICU in the group that maintained antimicrobials, without any deaths.
Conclusions
The reduction of antimicrobials in children with FN and respiratory viral infections, based on clinical and microbiological/molecular diagnostic criteria, should favour the adoption of evidence-based management strategies in this population.
Keywords: Antimicrobials, Cancer, Children, Febrile neutropenia, Respiratory viral infection
Introduction
Children with cancer are exposed to viral, bacterial and fungal infections, especially during the episodes of fever and neutropenia (FN) [1], [2], [3]. Episodes of FN during chemotherapy are currently managed within the hospital with broad-spectrum antimicrobial therapy [4], [5]. Until recently, the most common aetiological agents reported in FN episodes occurring in children with cancer have been bacterial and fungal pathogens. Viral infections, particularly respiratory viruses, have been increasingly recognized as significant aetiological agents of FN in this population [6], [7].
Respiratory viral infections are a leading cause of morbidity and mortality in immunocompetent children [8], [9]. In paediatric patients with cancer, respiratory viruses can be detected in up to 57% of episodes of FN [6], [7], [10], [11], [12], [13], [14], [15]. In a previous study we determined the frequency and clinical outcome of respiratory virus-positive FN episodes [6]. Respiratory viruses were the most common agents detected and clinical outcomes of these episodes were significantly better than episodes with single bacterial infections or viral–bacterial co-infections. Children with respiratory viral infections had fewer days of hospitalization, a lower probability of haemodynamic instability and lower rates of admission to the paediatric intensive care unit (PICU) [6].
For children with FN most research efforts have focused on management of bacterial and fungal infections, mainly in proposing models of risk prediction for invasive bacterial and fungal infections [16], [17], [18], [19], [20], [21], [22], [23], improvements in the molecular diagnosis of infections [24], [25] and selective antimicrobial management in children with high-risk and low-risk FN episodes [18], [26]. In contrast with the increase in studies of bacterial and fungal infections in this population, studies of viral infections are scarce and have been recognized as a research gap in the field [1]. A rational approach towards the management of a potential infection in children with cancer and FN requires a comprehensive analysis of all microbiological agents involved. Implementation of a systematic study and early detection of respiratory viral infection in children with cancer and FN might help to optimize their management by reducing hospitalization and antimicrobial use.
In this study we aim to determine the efficacy and safety of withholding antimicrobial treatment 48 h after admission in children with cancer and FN, with a demonstrated respiratory viral infection and a favourable clinical course.
Patients and methods
Population
From July 2012 to December 2015, a prospective, randomized, multicentre, government-sponsored study was conducted in five participating hospitals in Santiago, Chile, belonging to the National Child Programme of Antineoplastic Drugs network. Children and adolescents with cancer ≤18 years of age admitted with an episode of FN were enrolled after parental and child signed informed consent and assent (if older than 8 years of age). Children with haematopoietic stem cell transplants were excluded. This study was approved by the ethics committee of each participating institution.
Overall study design
Children were evaluated at admission to characterize the febrile episode, establish their risk for invasive bacterial infection and perform a common protocol for diagnosis of bacterial and viral pathogens. We recorded age, gender, type of cancer, type and date of the last chemotherapy, use of granulocyte colony-stimulating factor, use of antimicrobial prophylaxis, use of central venous catheter, hours of fever before admission, axillary temperature, blood pressure, heart rate, respiratory rate, signs and symptoms indicative of an infectious focus—especially respiratory signs/symptoms, haematological status (absolute neutrophil count (ANC), haemoglobin level, platelet count), biochemical tests, quantitative C reactive protein (CRP), central and peripheral automated blood cultures, other cultures if clinically indicated, and a molecular-based evaluation for respiratory viruses.
After initial evaluation, all children were treated following the Latin American Consensus for a Rational Approach of Children with FN [27] and Guidelines for the management of FN in children with cancer [1]. Briefly, children were hospitalized and low-risk FN episodes were treated with a third-generation cephalosporin (ceftriaxone) whereas children at high risk were treated with an anti-pseudomonal third-generation cephalosporin (ceftazidim) plus amikacin with or without an anti-Gram-positive β-lactam or glycopeptide antimicrobial therapy.
After 48 h of hospitalization (day 3) children with a nasopharyngeal sample positive for a respiratory virus, absence of any positive bacterial culture and with a favourable clinical evolution, were subject to a 1:1 simple randomization by the study coordinator (blinded) using statistical software (GraphPad Prism, version 6.01; GraphPad, San Diego, CA, USA) into two groups: maintenance of antimicrobials until the end of the febrile episode, and the intervention group in which antimicrobials were withdrawn. According to ethical committee requirements, it was possible to randomize each child in only one episode of FN that met the study criteria, for this reason, one randomized episode of FN was equivalent to one randomized patient.
Both groups were monitored daily for clinical and laboratory evolution until fever resolution and ANC ≥500/mm3. Children with a bacterial pathogen or children in whom all studies gave negative results continued their antimicrobial management according to current standard of care. In the antimicrobial withdrawal group, criteria for re-instalment antimicrobial therapy were: resurgence of fever, clinical worsening or new clinical and/or laboratory findings suggesting a bacterial infection. One blinded investigator evaluated all cases after discharge, deciding that outcome was uneventful or not, without access to information about the specific intervention of each child (see Definitions). In children that continued with antimicrobial therapy, the standard duration of therapy was 7 days. Antimicrobials were stopped at day 7 if children had a favourable evolution, with at least a full day without fever and two consecutives CRP values ≤40 mg/L. The ANC was not a criterion for stopping antimicrobial therapy.
Study endpoints
The primary endpoint between the group that maintained antimicrobials and the group with antimicrobial withholding was number (%) of episodes with uneventful resolution. The secondary endpoints were (a) number of days of fever, (b) number of days of hospitalization, (c) percentage of episodes that develop a demonstrated or probable invasive bacterial infection, (d) re-instalment of antimicrobial therapy, (e) sepsis during hospitalization, (f) admission to the PICU, (g) death.
Laboratory evaluation
Laboratory tests. Haematological, biochemical and microbiological tests were performed at each hospital according to standard techniques.
Molecular detection for respiratory viruses. Detection was performed on nasopharyngeal samples collected from all children at the time of admission (Copan™ flocked swabs, Brescia, Italy). The swab was inserted into a vial containing viral transport medium (UTM-RT, Copan™), transported to the central laboratory and stored at –80°C until analysis in the next 24 h after admission. Total nucleic acid was extracted (easyMAG NucliSens; BioMérieux, Durham, NC, USA) and tested for 17 respiratory viruses (influenzas A, B, C; parainfluenzas 1, 2, 3, 4a and 4b; respiratory syncytial virus (RSV) A and B; rhinovirus; adenovirus; echovirus; human bocavirus; coronavirus; human metapneumoviruses A and B) by multiplex-PCR low-density microarray, according to the manufacturer’s instructions (PneumoVir; Genomica, Madrid, Spain).
Definitions. (a) neutropenia: ANC ≤500/mm3; (b) fever: single axillary temperature ≥38.5°C, or ≥38°C in two measurements separated by at least 1 h; (c) high-risk FN: an FN episode with one of the following factors at the time of admission: (i) relapse of leukaemia as cancer type, (ii) hypotension or (iii) quantitative CRP ≥90 mg/L, or with the following two factors: (iv) ≤7 days between the last chemotherapy and the beginning of the fever and (v) platelet count ≤50 000/mm3; (d) low-risk FN: an FN episode without the above mentioned factors; (e) episodes with favourable evolution after 48 h of antimicrobial therapy: good clinical condition (subjective evaluation of the clinical condition by the attending physician), temperature ≤38°C, absence of an identifiable new clinical focus and CRP <90 mg/L; (f) uneventful resolution: favourable resolution of the FN episode according to the randomized intervention, without changes in the therapy in the group that maintain antimicrobials and without re-instalment in the group with antimicrobial withholding; (g) demonstrated/probable invasive bacterial infection: bacteraemia or isolation of bacteria from a normally sterile site, or clinical signs suggestive of a localized bacterial infection, with parenchymal involvement, with or without microbiological isolation; (h) sepsis: systemic inflammatory response syndrome, accompanied by abnormal tissue perfusion, caused by a presumptive bacterial infection, with or without microbiological confirmation, (i) respiratory viral infection: detection of at least one respiratory virus in nasopharyngeal sample by PCR; (j) upper/lower respiratory viral infection: infection of respiratory tract that clinically affects from the larynx toward proximal or beyond the larynx plus respiratory viral infection, respectively.
Sample size and statistical analysis
Sample size. The primary endpoint for this non-inferiority study was number (%) of episodes with uneventful resolution. Sample size was calculated based on the hypothesis that the frequency of occurrence of uneventful resolution was the same for both groups. Considering an unfavourable outcome of 10% as previously reported for this population, a maximum acceptable difference of 10% between groups, a type I error of 0.05 and a potency of 90%, we calculated that 134 episodes were required, 67 in the group that maintain antimicrobial therapy and 67 in the antimicrobial withholding group. The overall capacity of enrolment in the five study sites was estimated in 300 episodes per year (25/month). Our enrolment was estimated for a total of 1000 FN episodes in the study period.
Statistical analysis
Categorical variables were compared with chi-square test or Fisher exact test depending on the number of episodes per comparison group. Continuous variables were compared between the two groups according to their distribution(Student’s t or Mann–Whitney U test).The risk for an uneventful resolution was evaluated between the exposed and unexposed groups calculating the OR with the respective 95% CI. Statistical analyses were performed with the Sigma Stat 3.0 Program and Systat software, CA, USA.
Results
Population characteristics
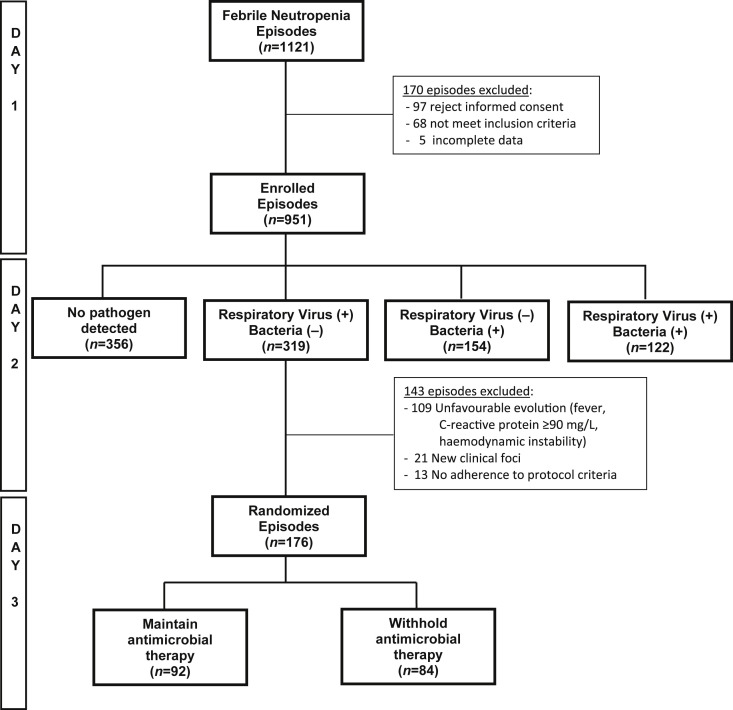
Between July 2012 and December 2015, a total of 1121 episodes of FN were evaluated in the five hospitals participating in the study, of which 951 (85%) episodes were enrolled and 170 (15%) were excluded, because 97/170 (57%) children and their parent refused to participate, 68/170 (40%) episodes did not meet the inclusion criteria and 5/170 (3%) had incomplete data. The 951 episodes enrolled were evaluated for bacterial and viral infections and all received empiric antimicrobial therapy. In all, 319 (34%) had a positive nasopharyngeal sample for respiratory viruses at admission and were negative for a bacterial pathogen. Forty-eight hours later, 176/319 (55%) of these episodes met all criteria for randomization, of which 92/176 (52%) were randomized into a current antimicrobial management and 84/176 (48%) into antimicrobial withholding. At the time of randomization, median ANC was 132/mm3 and 207/mm3 in the maintenance and withholding antimicrobial groups, respectively (p 0.15), with 87% and 80% of children having an ANC ≤500/mm3 in each group (p 0.3). From 143 episodes positive for a respiratory virus and negative for a bacterial pathogen not randomized, 109 did not have a favourable evolution after 48 h of antimicrobial therapy (104 maintain fever or CRP ≥90 mg/L, five had haemodynamic instability), 21 had new clinical foci and 13 were not randomized by no adherence to the protocol (Fig. 1 ).
Fig. 1.
Episodes of fever and neutropenia evaluated, enrolled and randomized during study period.
Table 1 describes the main characteristics of children with episodes of FN and a positive respiratory viral infection randomized to both groups. Age, gender, type of cancer and other characteristics were similar between the two groups (p NS). Less than 10% of children in each group received prophylaxis other than trimethoprim-sulfamethoxazole for Pneumocystis jirovecii. Eighty-eight of 176 episodes (50%) had a high-risk FN. Of the 176 episodes randomized, 65/92 (71%) and 66/84 (78%), respectively, had clinical characteristics suggesting an upper/lower respiratory tract infection at the time of admission.
Table 1.
Admission characteristics of 176 episodes of fever and neutropenia in children with a demonstrated respiratory viral infection, according to intervention
| Characteristics | Type of intervention |
p | |
|---|---|---|---|
| Maintain antimicrobial |
Antimicrobial withholding |
||
| n = 92 | n = 84 | ||
| Age in years, median (IQR) | 5 (3–9) | 4 (3–8) | 0.57 |
| Male gender, n (%) | 40 (44) | 46 (55) | 0.13 |
| Type of cancer, n (%) | |||
| Leukaemia/Lymphoma | 49 (53) | 55 (66) | 0.09 |
| Leukaemia relapse | 7 (8) | 4 (5) | 0.43 |
| Solid tumours | 36 (39) | 25 (29) | 0.19 |
| High risk for invasive bacterial infection, n (%) | 43 (47) | 45 (54) | 0.36 |
| Use of G-CSF, n (%) | 27 (29) | 26 (31) | 0.81 |
| Use of CVC, n (%) | 77 (84) | 68 (81) | 0.63 |
| Hours of fever previous to admission, median (IQR) | 2 (1–4) | 2 (1–3) | 0.10 |
| Admission clinical diagnosis, n (% ) | |||
| Fever without focus | 19 (20) | 13 (16) | 0.37 |
| Upper respiratory infection | 42 (46) | 44 (52) | 0.37 |
| Lower respiratory infection | 23 (25) | 22 (26) | 0.85 |
| Intestinal focus | 6 (7) | 5 (6) | 0.87 |
| Othersa | 2 (2) | 0 | |
G-CSF: granulocyte-colony stimulating factor; CVC: central venous catheter; IQR: Interquartile range
Others: Skin/soft tissue infection
Outcome of children with FN episodes by study groups
Table 2 shows the outcome of 176 randomized children, according to intervention. As expected, median durations of antimicrobial therapy were significantly different in children that maintained (7 days, interquartile range (IQR) 7–9 days) compared with children in whom antimicrobials were withdrawn (3 days, IQR 3–4 days), p <0.0001. Percentages of episodes of FN with uneventful resolution were 89/92 (97%) and 80/84 (95%), respectively (p NS), OR 1.48. (95% C.I. 0.32–6.83), p 0.61. Days of fever (2 and 1), days of hospitalization (6 and 6) and development of a demonstrated/probable invasive bacterial infection (2% and 1%) were similar among groups. No deaths occurred.
Table 2.
Outcome of 176 children with fever, neutropenia and a demonstrated respiratory viral infection, according to intervention
| Characteristics | Type of intervention |
Total | p | |
|---|---|---|---|---|
| Maintain antimicrobial |
Antimicrobial withholding |
|||
| n = 92 | n = 84 | |||
| Days of antimicrobial therapy, median (IQR) | 7 (7–9) | 3 (3–4) | 6 (3–7) | < 0.0001 |
| Days of fever after admission, median (IQR) | 2 (1–3) | 1 (1–2) | 1 (1–2) | 0.44 |
| Days of hospitalization, median (IQR) | 6 (4–8) | 6 (4–7) | 6 (4–7) | 0.65 |
| Days of ANC <500/mm3, median (IQR) | 5 (3–8) | 4 (3–8) | 5 (3–8) | 0.23 |
| Days of AMC <100/mm3, median (IQR) | 3 (0–6) | 2 (0–5) | 3 (0–5) | 0.46 |
| Resolving uneventfully, n (%) | 89 (97) | 80 (95) | 169 (96) | 0.61 |
| Demonstrated/probable invasive bacterial infection, n (%) | 2 (2) | 1 (1) | 3 (2) | 0.93 |
| Re-instalment of antimicrobials, n (%) | 4 (5) | 4 (2) | ||
| Development of sepsis, n (%) | 1 (1) | 0 | 1 (1) | |
| Admission to PICU, n (%) | 1 (1) | 0 | 1 (1) | |
| Death, n (%) | 0 | 0 | 0 | |
Abbreviations: AMC, absolute monocyte count; ANC, absolute neutrophil count; IQR: Interquartile range; PICU, paediatric intensive care unit.
Three children had a demonstrated/probable bacterial infection, two had bacteraemia caused by extended spectrum β-lactamase-positive Klebsiella pneumoniae in the group maintaining antimicrobials and one case of a culture-negative sinusitis in the antimicrobial withholding group. Re-instalment of antimicrobial therapy was indicated in four children. All four had acute myeloid leukaemia, were admitted with a high-risk FN and met study criteria for upper respiratory viral infection. In the four cases where antimicrobials were discontinued with children in good clinical conditions, temperature <38°C and CRP <90 mg/L, according to the protocol; three of them presented with fever between days 5 and 7 after admission, without identifiable new foci or clinical deterioration and with CRP <90 mg/L. The clinical course was favourable in three of the children with fever declining after one day of new antimicrobial therapy, in the absence of any bacterial pathogen detection. The fourth child developed fever on day 5 and an increase in CRP to 100 mg/L. Clinical and imaging findings suggested sinusitis requiring a 14-day antimicrobial therapy. The child did not develop sepsis and was not admitted to PICU. He was discharged with a diagnosis of probable invasive bacterial infection.
One child from the group that maintained antimicrobials developed sepsis and was admitted to the PICU. She was a 3-year-old girl with a neuroblastoma, a high-risk FN and an upper respiratory infection. On the day of randomization, she was afebrile and CRP value was 14 mg/L. On day 6 she presented with fever and haemodynamic instability. Blood cultures obtained at day 6 were positive for an extended spectrum β-lactamase-positive Klebsiella pneumoniae, CRP value increased up to 250 mg/L and she was admitted to PICU receiving antimicrobials based on the bacterial isolation for a new period of 14 days, with good clinical outcome. No deaths occurred among the 176 randomized children during the study period.
We performed a separated analysis of the 176 episodes randomized according to the risk status for invasive bacterial infection determined at admission. For the 88 children with episodes of low-risk FN the median durations of antimicrobial therapy were 7 days (IQR 7–9) and 3 days (IQR 3–3), p <0.001; with 98% and 100% of episodes with uneventful resolution, without cases of antimicrobial re-instalment, development of sepsis and admission to PICU. In the 88 episodes of high-risk FN, the median durations of antimicrobial therapy were 7 days (IQR 7–9) and 4 days (IQR 3–5), p <0.001, with 93% of episodes with uneventful resolution in both groups, with the four cases of antimicrobial re-instalment and one case of sepsis and admission to PICU, all of them described previously.
Respiratory viruses identified in each study group
The main respiratory viruses identified in the 176 children randomized with episodes of FN are described in Table 3 . Overall, the most common respiratory virus was rhinovirus (41%) followed by RSV, parainfluenza virus and influenza virus with no differences observed between groups. These four viruses represented 88% of the identified respiratory viruses in both groups. We detected 23 and 22 cases of viral co-infection in each group. In 43/45 cases (96%) viral co-infection included two respiratory viruses. Three of the four children requiring antimicrobial re-instalment had RSV and one of them had a rhinovirus. The child that developed a Klebsiella pneumoniae sepsis had a positive parainfluenza virus detection.
Table 3.
Respiratory viruses identified in 176 children with episodes of fever and neutropenia, according to intervention
| Respiratory viruses | Type of intervention |
Total c | |
|---|---|---|---|
| Maintain antimicrobial a |
Antimicrobial withholding b |
||
| n = 92 | n = 84 | ||
| Rhinovirus | 49 (53) | 42 (50) | 91 (41) |
| Respiratory sincytial virus | 17 (19) | 24 (29) | 41 (19) |
| Parainfluenza virus | 21 (23) | 18 (21) | 39 (18) |
| Influenza virus | 14 (15) | 8 (10) | 22 (10) |
| Metapneumovirus | 4 (4) | 5 (6) | 9 (4) |
| Adenovirus | 5 (5) | 2 (2) | 7 (3) |
| Bocavirus | 5 (5) | 5 (6) | 10 (5) |
| Coronavirus | 1 (1) | 1 (1) | 2 (1) |
Twenty-three episodes with viral co-infection. Twenty-one cases with two respiratory viruses and two cases with three respiratory viruses.
Twenty-two episodes with viral co-infecction. All cases with two respiratory viruses.
Without significant differences between groups.
Discussion
Respiratory viruses were detected as sole pathogens in one-third of children with cancer admitted because of a febrile neutropenic episode, of which almost half met study criteria for favourable evolution at 48 h, allowing their randomization. Overall, 95% of the episodes in which antimicrobials were withdrawn had an uneventful resolution.
We previously demonstrated that it was possible to apply selective management, proposing ambulatory therapy in children with low-risk FN, defined according to the following parameters: type of cancer, haemodynamic stability, CRP values, platelet count and number of days since the last chemotherapy [26]. In this study, we advance a step further, including the possibility of stopping antimicrobial therapy 48 h after admission in children with a positive respiratory viral infection, a negative bacterial infection and a favourable clinical course, including episodes with low and high risk for invasive bacterial infection.
Knowledge on the epidemiology of respiratory viral infection in immunocompromised children is scarce with few systematic studies aimed to determine their clinical impact [6]. RSV, influenza virus, parainfluenza virus, adenovirus and picornaviruses have been the most common aetiological agents detected in this population [6], [7], [12]. The increasing detection of viruses highlights the relevance of optimizing viral diagnosis in children with FN [1].
Children with cancer and high-risk FN require immediate hospitalization and aggressive intravenous antimicrobial treatment. We observed an uneventful resolution in 96% of all randomized FN episodes, despite the fact that 50% of them were classified as high risk at admission. Considering these data, systematic evaluation for respiratory viruses at the time of hospital admission could be helpful to establish a more rational treatment in a selected group of patients. Miedema et al. [18] recently reported that it is safe to shorten antimicrobial treatment to 72 h in selected medium-risk children with FN; antimicrobials were stopped in 50 episodes and no failures were observed, however, no high-risk episodes were included in this study.
Approximately 79% of episodes of FN do not present a serious bacterial infection [28], therefore a more extensive assessment with multiplex blood and respiratory PCR assays could increase pathogen detection [6], [24], [29], facilitating a tailor-made therapy and reducing unnecessary antimicrobial exposure [3].
This study had limitations, including that the intervention was not double blind. We decided not to conduct a double-blind study because the majority of these children received antimicrobial therapy using their central venous catheter. We preferred not to use the central venous catheter in children with antimicrobials withheld to avoid unnecessary manipulations. Another possible issue is that not all children randomized in this cohort with respiratory virus detection presented an upper or lower respiratory tract infection at the time of admission. Although positive PCR detection is not always necessarily related with respiratory disease [30], the majority of children in this study (75%) presented respiratory symptoms, suggesting a role for the respiratory viruses detected by PCR.
In conclusion, we suggest incorporating molecular respiratory viral study in the initial evaluation of all children with cancer and episodes of FN. The reduction of antimicrobial use in children with FN with a demonstrated respiratory viral infection, based on stringent clinical and microbiological/ molecular diagnostic criteria, could favour the adoption of evidence-based and more rational management strategies in this population.
Transparency declaration
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the National Fund for Scientific and Technological Development (FONDECYT), Chile (grants numbers 1120800, 1130911).
Acknowledgements
We thank research nurses of the participant hospitals for their invaluable support in enrolling patients and obtaining blood and nasopharyngeal samples. We appreciate the support provided by Magdalena Castro, RN and clinical epidemiologist in the statistical analysis. We thank the FONDECYT Programme for their support.
Editor: L. Leibovici
References
- 1.Lehrnbecher T., Phillips R., Alexander S., Alvaro F., Carlesse F., Fisher B. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30:4427–4438. doi: 10.1200/JCO.2012.42.7161. [DOI] [PubMed] [Google Scholar]
- 2.Hagag A.A., Hassan S.M., Elgamasy M.A., Afifi I.K. Study of Common Bacterial and Fungal Pathogens in Children with Hematological Malignancies during Febrile Neutropenia: Single Center Egyptian Study. Infect Disord Drug Targets. 2016;16:54–62. doi: 10.2174/1871526516666151230124333. [DOI] [PubMed] [Google Scholar]
- 3.Barton C.D., Waugh L.K., Nielsen M.J., Paulus S. Febrile neutropenia in children treated for malignancy. J Infect. 2015;71(Suppl. 1):S27–S35. doi: 10.1016/j.jinf.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Santolaya M.E. Supportive care in children. Curr Opin Oncol. 2010;22:323–329. doi: 10.1097/CCO.0b013e32833a8752. [DOI] [PubMed] [Google Scholar]
- 5.Hughes W.T., Armstrong D., Bodey G.P., Bow E.J., Brown A.E., Calandra T. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 6.Torres J.P., Labrana Y., Ibanez C., Kasaneva P., Farfan M.J., De la Maza V. Frequency and clinical outcome of respiratory viral infections and mixed viral-bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J. 2012;31:889–893. doi: 10.1097/INF.0b013e31825c4b7e. [DOI] [PubMed] [Google Scholar]
- 7.Benites E.C., Cabrini D.P., Silva A.C., Silva J.C., Catalan D.T., Berezin E.N. Acute respiratory viral infections in pediatric cancer patients undergoing chemotherapy. J Pediatr (Rio J) 2014;90:370–376. doi: 10.1016/j.jped.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukutani K.F., Nascimento-Carvalho C.M., Van der Gucht W., Wollants E., Khouri R., Dierckx T. Pathogen transcriptional profile in nasopharyngeal aspirates of children with acute respiratory tract infection. J Clin Virol. 2015;69:190–196. doi: 10.1016/j.jcv.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meissner H.C. Viral Bronchiolitis in Children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 10.Christensen M.S., Nielsen L.P., Hasle H. Few but severe viral infections in children with cancer: a prospective RT-PCR and PCR-based 12-month study. Pediatr Blood Cancer. 2005;45:945–951. doi: 10.1002/pbc.20469. [DOI] [PubMed] [Google Scholar]
- 11.Koskenvuo M., Mottonen M., Rahiala J., Saarinen-Pihkala U.M., Riikonen P., Waris M. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J. 2008;27:974–980. doi: 10.1097/INF.0b013e31817b0799. [DOI] [PubMed] [Google Scholar]
- 12.Lindblom A., Bhadri V., Soderhall S., Ohrmalm L., Wong M., Norbeck O. Respiratory viruses, a common microbiological finding in neutropenic children with fever. J Clin Virol. 2010;47:234–237. doi: 10.1016/j.jcv.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendoza Sanchez M.C., Ruiz-Contreras J., Vivanco J.L., Fernandez-Carrion F., Baro Fernandez M., Ramos J.T. Respiratory virus infections in children with cancer or HIV infection. J Pediatr Hematol Oncol. 2006;28:154–159. doi: 10.1097/01.mph.0000210061.96075.8e. [DOI] [PubMed] [Google Scholar]
- 14.Martinez P., Cordero J., Valverde C., Unanue N., Dalmazzo R., Piemonte P. Viral respiratory co-infections in pediatric patients admitted for acute respiratory infection and their impact on clinical severity. Rev Chilena Infectol. 2012;29:169–174. doi: 10.4067/S0716-10182012000200008. [DOI] [PubMed] [Google Scholar]
- 15.Wong-Chew R.M., Espinoza M.A., Taboada B., Aponte F.E., Arias-Ortiz M.A., Monge-Martinez J. Prevalence of respiratory virus in symptomatic children in private physician office settings in five communities of the state of Veracruz, Mexico. BMC Res Notes. 2015;8:261. doi: 10.1186/s13104-015-1239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santolaya M.E., Alvarez A.M., Becker A., Cofre J., Enriquez N., O'Ryan M. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19:3415–3421. doi: 10.1200/JCO.2001.19.14.3415. [DOI] [PubMed] [Google Scholar]
- 17.Santolaya M.E., Alvarez A.M., Aviles C.L., Becker A., Cofre J., Enriquez N. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clin Infect Dis. 2002;35:678–683. doi: 10.1086/342064. [DOI] [PubMed] [Google Scholar]
- 18.Miedema K.G., Tissing W.J., Abbink F.C., Ball L.M., Michiels E.M., van Vliet M.J. Risk-adapted approach for fever and neutropenia in paediatric cancer patients—a national multicentre study. Eur J Cancer. 2016;53:16–24. doi: 10.1016/j.ejca.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Spasova M.I., Grudeva-Popova J.G., Kostyanev S.S., Genev E.D., Stoyanova A.A., Kirina V.I. Risk index score for bacteremia in febrile neutropenic episodes in children with malignancies. J BUON. 2009;14:411–418. [PubMed] [Google Scholar]
- 20.Villarroel M., Aviles C.L., Silva P., Guzman A.M., Poggi H., Alvarez A.M. Risk factors associated with invasive fungal disease in children with cancer and febrile neutropenia: a prospective multicenter evaluation. Pediatr Infect Dis J. 2010;29:816–821. doi: 10.1097/INF.0b013e3181e7db7f. [DOI] [PubMed] [Google Scholar]
- 21.Ozsevik S.N., Sensoy G., Karli A., Albayrak C., Dagdemir A., Belet N. Invasive fungal infections in children with hematologic and malignant diseases. J Pediatr Hematol Oncol. 2015;37:e69–e72. doi: 10.1097/MPH.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 22.Santolaya M.E., Alvarez A.M., Aviles C.L., Becker A., Venegas M., ÓRyan M. Prospective validation of a risk prediction model for severe sepsis in children with cancer and high risk fever and neutropenia. Pediatr Infect Dis J. 2013;32:1318–1323. doi: 10.1097/01.inf.0000436128.49972.16. [DOI] [PubMed] [Google Scholar]
- 23.Rondinelli P.I., Ribeiro Kde C., de Camargo B. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol. 2006;28:665–670. doi: 10.1097/01.mph.0000212996.94929.0b. [DOI] [PubMed] [Google Scholar]
- 24.Santolaya M.E., Farfan M.J., De La Maza V., Cocina M., Santelices F., Alvarez A.M. Diagnosis of bacteremia in febrile neutropenic episodes in children with cancer: microbiologic and molecular approach. Pediatr Infect Dis J. 2011;30:957–961. doi: 10.1097/INF.0b013e31822a37d7. [DOI] [PubMed] [Google Scholar]
- 25.Shachor-Meyouhas Y., Sprecher H., Moscoviz D., Zaidman I., Haimi M., Kassis I. Molecular-based diagnosis of bacteremia in the setting of fever with or without neutropenia in pediatric hematology-oncology patients. J Pediatr Hematol Oncol. 2013;35:500–503. doi: 10.1097/MPH.0b013e31829eec78. [DOI] [PubMed] [Google Scholar]
- 26.Santolaya M.E., Alvarez A.M., Aviles C.L., Becker A., Cofre J., Cumsille M.A. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. J Clin Oncol. 2004;22:3784–3789. doi: 10.1200/JCO.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 27.Paganini H., Santolaya M.E., Alvarez M., Araña M., Arteaga R., Bonilla A. Diagnóstico y tratamiento de la neutropenia febril en niños con cáncer: Consenso de la Sociedad Latinoamericana de Infectología Pediátrica. Rev chil infectol. 2011;28(Suppl. 1):10–38. doi: 10.4067/s0716-10182021000600857. [DOI] [PubMed] [Google Scholar]
- 28.Castagnola E., Fontana V., Caviglia I., Caruso S., Faraci M., Fioredda F. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 29.Babady N.E., Mead P., Stiles J., Brennan C., Li H., Shuptar S. Comparison of the Luminex xTAG RVP Fast assay and the Idaho Technology FilmArray RP assay for detection of respiratory viruses in pediatric patients at a cancer hospital. J Clin Microbiol. 2012;50:2282–2288. doi: 10.1128/JCM.06186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]