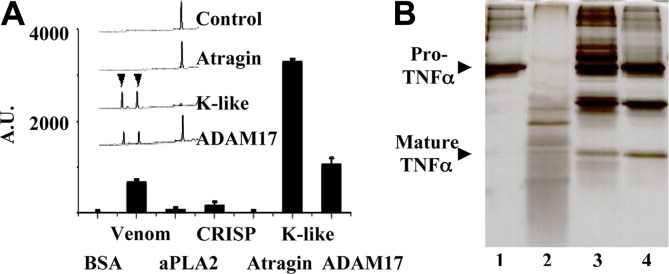
Fig. 3.
The enzymatic effect of atragin and K-like on Pro-TNFα. (A) Fluorometric measurements of the pro-TNFα-derived peptide (Abz-LAQAVRSSSR-Dpa) cleavage in the presence of various snake venom components. Both crude venom and purified K-like SVMP show a TACE activity as great as ADAM17 but not the other venom proteins. The HPLC profile (inserted panel) of the peptide fragments digested with the metalloprotease indicates that the specificity of K-like on a TNFα-derived peptide is identical to that of ADAM17. (The cleavage site of the peptide was identified with mass spectra.) (B) The SDS–PAGE analysis of cleavage patterns of pro-TNFα fusion substrates with two SVMPs. Lane 1: the pro-TNFα fusion substrate for the control; lane 2: pro-TNFα randomly digested by atragin; lane 3: TNFα released by K-like; lane 4: TNFα released by ADAM17.