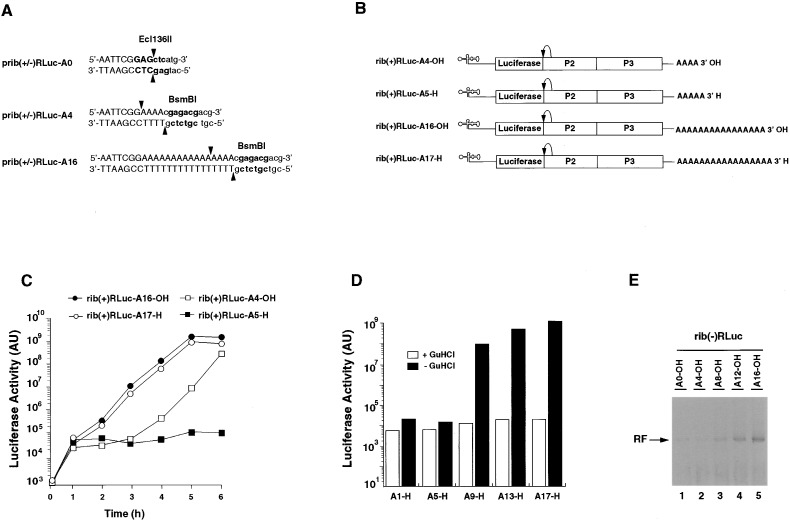
Figure 2.
A Minimum Poly(A) Tail Is Necessary for RNA Replication In Vivo and In Vitro
(A) Restriction endonuclease recognition sites used for linearization of replicon-encoding cDNAs to generate transcripts with defined 3′ ends. Recognition sites are shown in bold, cleavage sites are indicated by arrows. Transcribed regions are shown in uppercase letters.
(B) Schematic presentation of the replicon RNAs with modified 3′ ends. Rib(+)Rluc RNA with 4 or 16 adenylates at their 3′ ends. In rib(+)RLuc-A5-H and -A17-H, the 3′ end were blocked with a 3′ deoxyadenylate.
(C) Transcripts with blocked 3′ ends and short poly(A) tails do not replicate in vivo. Replicon transcripts were transfected into 293 cells and incubated at 37°C. Luciferase activity in the transfected cells was determined at the indicated time points.
(D) A minimum poly(A) tail containing 8 adenylate residues is necessary for replication. Luciferase expressing replicon RNAs with 0, 4, 8, 12, or 16 adenylate residues and a single 3′-dA at their 3′ ends were transfected into 293 cells. The cells were incubated at 37°C in the absence or presence of 2 mM GuHCl, and luciferase activity was determined at 5 hr posttransfection.
(E) A minimum poly(A) tail containing 8 adenylate residues is necessary for efficient negative strand RNA synthesis in vitro. Replicon rib(−)RLuc RNAs with 0, 4, 8, 12, or 16 adenylate residues at their 3′ ends were used to program an in vitro translation/replication extract. The radiolabeled products of the reaction were analyzed on native 0.8% agarose gels and visualized by autoradiography