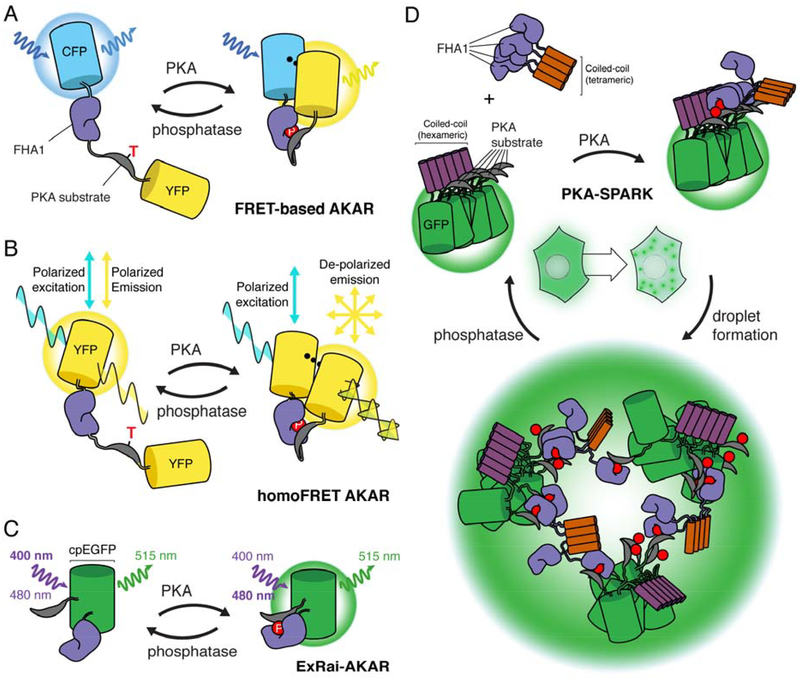
Figure 1: Design of genetically encoded kinase activity reporters for PKA (AKAR).
A, the design of FRET-based AKAR. The FRET pair cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) flank the phosphoamino acid binding domain, forkhead associated domain 1 (FHA1), and a PKA-specific substrate, with the threonine subject to phosphorylation highlighted. Upon PKA phosphorylation of the biosensor, a phosphatase-reversible conformational change is induced which alters the FRET efficiency. B, design of homoFRET AKAR. Two identical fluorescent proteins, YFP, flank the sensing component of AKAR, with polarized excitation and emission. Upon PKA phosphorylation and biosensor conformational change, YFP emission becomes de-polarized, which is used as the readout of PKA activity. C, design of excitation-ratiometric AKAR (ExRai AKAR), where circularly permutated EGFP (cpEGFP) is between the PKA substrate and FHA1 domains. PKA phosphorylation or phosphatase activity induce conformational changes in ExRai AKAR, resulting in switching of the excitation wavelength of cpEGFP from ~400 nm when the biosensor is unphosphorylated to ~480 nm when the biosensor is phosphorylated. D, design of separation of phases-based activity reporter of kinase (SPARK) for PKA (PKA-SPARK). The PKA-specific substrate is tethered to GFP and a hexameric coiled-coil domain, whereas the FHA1 domain is attached to a tetrameric coiled-coil. Upon PKA phosphorylation, FHA1 binds to the phosphorylated PKA substrate, resulting in phase-separated liquid droplets, which can be reversed by phosphatase activity.