Figure 4.
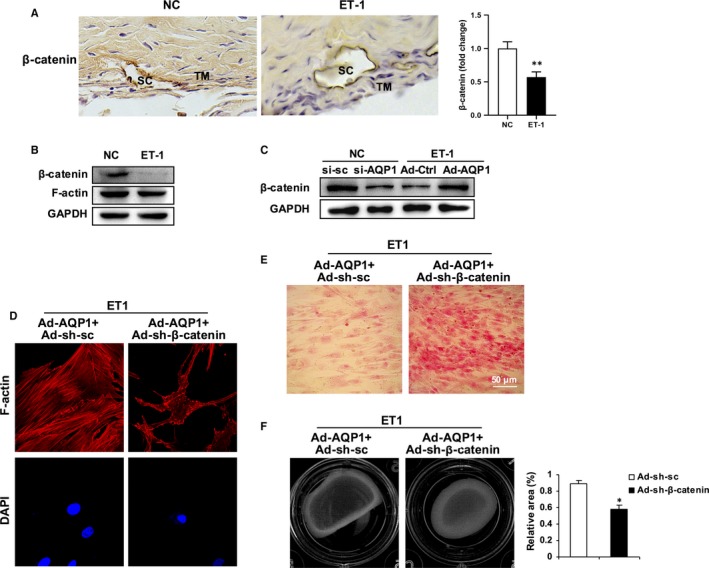
β‐catenin is required for the role of AQP1 in inhibiting ET‐1‐induced TM remodelling. A, Representative immunohistochemistry for β‐catenin in sections of rabbit trabecular meshwork tissues. TM, trabecular meshwork; SC, Schlemm's canal. B, Western blot analysis for β‐catenin and F‐actin in PBS or ET‐1 (100 nmol/L, 24 h) treated HTMCs. C, HTMCs were transfected with si‐AQP1 or scramble control si‐sc for 48 h in control HTMCs. Alternatively, cells were infected with an adenoviral vector expressing AQP1 (Ad‐Flag‐AQP1) or empty vector (Ad‐Flag). Forty‐eight hours later, cells were treated with ET‐1 for another 24 h. Protein level of β‐catenin was determined by Western blot. D‐F, HTMCs were co‐infected with an adenoviral vector expressing Ad‐AQP1 (Ad‐Flag‐AQP1) and an adenoviral vector expressing short hairpin RNA specific for β‐catenin (Ad‐sh‐β‐catenin) or scramble control (Ad‐sh‐sc). Forty‐eight hours later, cells were treated with ET‐1 for another 24 h. D, Representative immunofluorescence for F‐actin (red). Nuclei were counterstained with DAPI (blue). Scale bar, 10 μm. E, Deposition of collagen in HTMCs was determined by Sirius Red staining. F, The contractility of HTMCs was determined by a collagen gel contraction assay. Representative images are shown. The area of the collagen disc was measured using Image J. The contraction degree was expressed as a percentage of the 24‐well area. The cultured HTMCs are from one human donor. Data are means ± SEM of three independent experiments. **P < .01 vs control
