Abstract
Purpose
Evidence suggests that chronic obstructive pulmonary disease (COPD) symptoms and progression may differ between men and women. However, limited information is currently available on the pathophysiological and biological factors that may underlie these sex-related differences. The objective of this review is to systematically evaluate reports of potential sex-related differences, including genetic, pathophysiological, structural, and other biological factors, that may influence COPD development, manifestation, and progression in women.
Patients and Methods
A PubMed literature search was conducted from inception until January 2020. Original reports of genetic, hormonal, and physiological differences, and biological influences that could contribute to COPD development, manifestation, and progression in women were included.
Results
Overall, 491 articles were screened; 29 articles met the inclusion criteria. Results from this analysis demonstrated between-sex differences in inflammatory, immune, genetic, structural, and physiological factors in patients with COPD.
Conclusion
Various biological differences are observed between men and women with COPD including differences in inflammatory and metabolic pathways related to obesity and fat distribution, immune cell function and autophagy, extent and distribution of emphysema and airway wall remodeling. An enhanced understanding of these differences has the potential to broaden our understanding of how COPD develops and progresses, thereby providing an opportunity to ultimately improve diagnosis, treatment, and monitoring of COPD in both men and women.
Keywords: COPD, biological, sex, systematic review, women
Introduction
Chronic obstructive pulmonary disease (COPD) was, until recently, largely considered a disease of elderly men who smoke.1,2 However, as our understanding has evolved, COPD is increasingly being viewed as a disease that greatly impacts women as well, especially with the increased prevalence of smoking among women.3–6 A meta-analysis of 156 studies reported that globally, in 2015, an estimated 9.23% of the men and 6.16% of the women had COPD.7 While gender-wise prevalence varied widely among the different World Health Organization Global Burden of Disease subregions, the highest prevalence among women, at 7.30%, was in North America.7 A concerning shift toward an increase in the incidence and prevalence of COPD in women under 60 years of age has also been noted.8,9 This rising prevalence of COPD among women also means that more women are dying of COPD, with global projections indicating that women are at a greater risk of dying because of COPD than breast and lung cancer combined.10 A 2016 report from the Centers for Disease Control and Prevention revealed a decline in the age-adjusted COPD-related death rate for both men and women from 2000 through 2014, but the decline was more rapid among men.11
Various behavioral, environmental, sociocultural, and clinical factors have likely contributed to the rising prevalence of COPD in women. One of the greatest risk factors for COPD in the developed world is cigarette smoking,2 which peaked among women in the US in the 1980s.6 Other risk factors for COPD in women include exposure to biomass smoke—especially in women from developing countries;12,13 occupational exposure to textiles, ceramics, glassware, and brassware;14 and respiratory infections, such as tuberculosis.15,16 Notably, women also comprise the majority of never-smokers who develop COPD, further suggesting gender differences in risk factors for disease development.17
Data suggest that disease progression and presentation may also differ between the sexes.9,18–21 A meta-analysis of population-based cohort studies reported that female current smokers, with increasing age, experience accelerated annual decline in forced expiratory volume in 1 second (FEV1) compared with male current smokers.21 This is corroborated by data from a recently published large cohort study in the United Kingdom, which showed that women had a greater risk of airflow obstruction than men despite exposure to similar tobacco dose.22 In the cross-sectional, population-based, Proyecto Latinoamericano de Investigación en Obstrucción Pulmonar (PLATINO) study, women reported more dyspnea and greater physical limitations than men.18 Multiple studies have also demonstrated more frequent exacerbations among women with COPD than men with COPD.23–25 Together these findings provide strong evidence for differences in clinical presentation and manifestation of COPD between the sexes. However, the underlying cause driving these sex-specific differences remains largely unknown. Few studies have been conducted to understand the pathophysiological causes of the differences between men and women with COPD. Consequently, the objective of this analysis was to systematically evaluate reports of potential sex-related factors, including genetic, pathophysiological, and structural differences, as well as other biological factors, that may influence COPD development, manifestation, and progression in women.
Methods
Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.26 A PubMed search was conducted from inception until January 9, 2020, for articles using the following terms: (COPD[title] OR pulmonary disease, chronic obstructive[title] OR emphysema[title]) AND (women[title] OR female[title] OR men[title] OR male[title] OR gender[title] OR sex[title]). Literature search results were limited to articles published in English. The reference lists of articles identified from this search were also reviewed to identify any other relevant studies.
Study Selection
Articles retrieved from the PubMed search were imported into an EndNote library. Titles and abstracts were screened by Suchita Nath-Sain (SNS) and Maribeth Bogush (MB) and independently verified by MeiLan K Han (MKH). Articles were included if they were original reports of genetic, hormonal, and physical differences (eg, airway and lung size) and biological influences that could contribute to COPD development, manifestation, and progression in women. Narratives and systematic reviews, letters to the editor, congress reports, editorials, errata, withdrawals, newsletters, and articles that focused on men or animal models, or those that reported on adherence, asthma-COPD overlap, clinical characteristics, comorbidities, epidemiology, healthcare costs, hospitalizations, monitoring, risks associated with COPD, quality of life, and underdiagnoses were excluded.
After application of the inclusion and exclusion criteria, full texts of the remaining articles (and their reference lists) were reviewed by SNS and MB to identify articles for this analysis. Categorization of articles meeting the inclusion or exclusion criteria and relevant data extraction were performed by SNS and MB and independently reviewed by MKH. Any disagreements were resolved by consensus-based discussions.
Results
Study Selection
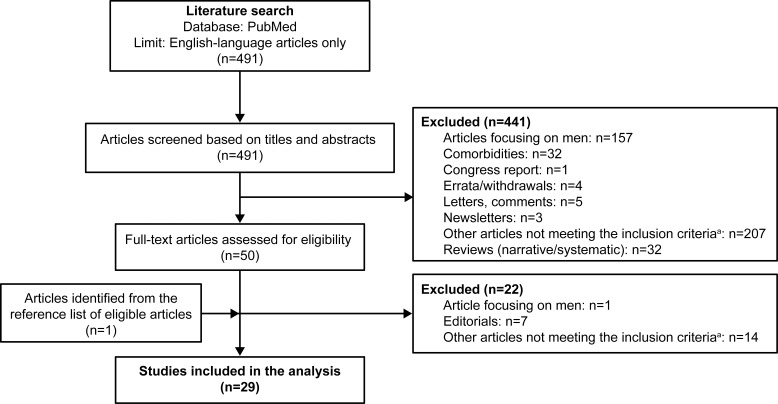
Overall, the PubMed search yielded 491 relevant articles (Figure 1). After review of the titles and abstracts of all the articles, 441 articles were excluded from the analysis as they did not meet the inclusion criteria. Of these, 35.8% (n=157) were studies in men alone and 7.3% (n=32) were review articles. A total of 50 articles were considered for full-text review, including 12 articles for which no abstracts were available. After a full-text review, 29 articles were included in the analysis (Supplementary Table 1); of these, 1 was added following a review of the reference lists of eligible articles.
Figure 1.
Flowchart of the included studies.
Notes: aArticles reporting adherence, asthma-COPD overlap, clinical characteristics, epidemiology, healthcare costs, hospitalizations, monitoring, risks associated with COPD, quality of life, animal models, and underdiagnoses.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Summary of Study Results
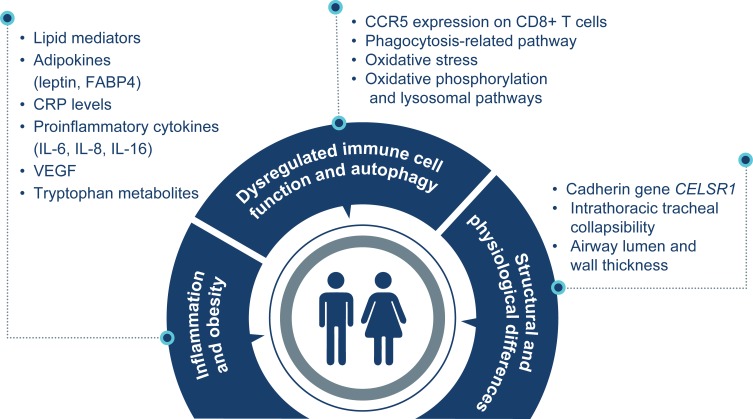
The studies included in this analysis included 298,975 healthy individuals or patients with COPD. Overall, results from this analysis demonstrated between-sex differences in inflammatory, immune, genetic, structural, and physiological factors in patients with COPD (Supplementary Table 1; Figure 2).
Figure 2.
Sex differences in COPD.
Abbreviations: CCR, CC chemokine receptor; CD, cluster of differentiation; CELSR1, cadherin EGF LAG seven-pass G-type receptor 1; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FABP4, fatty acid‒binding protein 4; IL, interleukin; VEGF, vascular endothelial growth factor.
Inflammation and Obesity
COPD is characterized by chronic inflammation of the lungs – especially in the lung parenchyma and peripheral airways – as well as systemic inflammation, which may contribute to comorbidities.27 Several inflammatory mediators, including lipid mediators, play an important role in the inflammatory pathways involved in COPD.27,28 As such, studies have been conducted to understand the role of lipid mediators in sex-related differences in COPD.29,30 Multivariate modeling of COPD-related lipid mediator levels in bronchoalveolar lavage fluid (BALF) samples obtained from the Karolinska COSMIC (Clinical & Systems Medicine Investigations of Smoking-related Chronic Obstructive Pulmonary Disease) cohort, which comprised healthy never-smokers, smokers with normal lung function, and patients with COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 1–2/A–B), identified a 9-lipid panel that differentiated female smokers with COPD from those with normal lung function.29 The panel included mediators from the linoleic acid–derived cytochrome P450 (CYP) pathway, as well as arachidonic acid–derived products of thromboxane synthase and 5-lipoxygenase. Notably, this difference was not observed among men, suggesting a female-dominated disease subphenotype.
Adipokines are cytokines secreted by adipose tissue.31 Leptin, a pro-inflammatory cytokine, and adiponectin, an anti-inflammatory cytokine, have been implicated in the pathogenesis of COPD.31 Circulating leptin levels increase in women with COPD but not in men with COPD or healthy women.30 Moreover, circulating leptin levels correlated with plasma C-reactive protein (CRP) levels – a marker of systemic inflammation – in women with COPD, but not in men.30 Leptin is produced by adipose tissue and is believed to be important in regulating body weight.32 In individuals without COPD, leptin levels are higher in women at any given measure of obesity33,34 suggesting that sex-based differences in fat distribution may contribute to this finding and could also drive excess inflammation in women with COPD. Another adipokine, fatty acid–binding protein 4 (FABP4), which is an intracellular lipid chaperone playing a role in the regulation of inflammation, has also been found in higher levels in women than in men with COPD.35 Notably, a positive correlation between plasma FABP4 and CRP levels has been found in women with COPD. Hence, it is possible that sex-driven differences in adipokines could contribute to clinical phenotypic differences observed between men and women with COPD. Both leptin and adiponectin have been associated with lung function decline36 and exacerbations in patients with COPD.37
Data from ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points), a multicenter, observational COPD study, also demonstrated a gender association between body composition and inflammatory mediators in COPD.38 Computed tomography (CT) scan of chest of smokers (≥10 pack-years) with COPD showed that women, compared with men, had a significantly lower pectoralis muscle area (PMA) and a higher subcutaneous adipose tissue (SAT) area.38 Moreover, SAT area was directly associated with CRP and fibrinogen levels in women, while PMA was not associated with any biomarker in either sex.38 In a cross-sectional study of patients with COPD, between-sex differences were observed in the plasma levels of pro-inflammatory cytokines (interleukin [IL]-6 and IL-16) and injury and repair (vascular endothelial growth factor [VEGF]).39 Although the plasma biomarker levels were similar in men and women smokers without COPD, significantly lower levels of IL-6 and VEGF and higher levels of IL-16 were observed in women vs men with COPD.39 Furthermore, higher IL-16 levels were associated with greater body mass index (BMI) in women with COPD, whereas VEGF levels were associated with markers of lung hyperinflation and emphysema only in men.39 While these data suggest that greater BMI in women with COPD may be associated with higher levels of inflammation, other data suggest lower BMI may also be associated with increased inflammation for women with COPD. In a separate study of malnourished men and women with COPD, differences in the serum concentrations of inflammatory cells and inflammatory markers were observed, with circulating neutrophils being significantly more abundant and mean CRP levels higher in malnourished women than men.40 Furthermore, there was a trend towards higher levels of the neutrophil-mobilizing cytokines IL-6 and IL-8 in malnourished women compared with men.40 Similarly, another study showed higher levels of pro-inflammatory cytokines including tumor necrosis factor-alpha and IL-8 in women with COPD than men. Moreover, women with COPD had a higher prevalence of signs of type II fiber atrophy (smaller cross-sectional area) and lower quadriceps muscle strength compared to men.41
Other studies have examined the relationship between circulating inflammatory markers and clinical outcomes. Two studies investigated the association between CRP levels and lung function.42,43 In participants from the European Community Respiratory Health Survey, higher CRP levels were significantly associated with reduced lung function (FEV1) in both men and women, although the decline in FEV1 was significantly higher in men than in women.43 In the Swiss Study on Air Pollution And Lung Disease In Adults (SAPALDIA), weight gain and rapid FEV1 decline were associated with elevated levels of high-sensitivity CRP; the association was significant in women but not in men. In totality, these data suggest that weight gain may be a greater risk factor for increased inflammation and COPD disease severity, particularly in women with COPD.42
Dysregulated Immune Cell Function and Autophagy
T lymphocytes are an important component of the adaptive immune system and their levels are higher in patients with COPD.44 T lymphocytes are directed to the site of inflammation when chemokines expressed on their surface bind to chemokine receptors.44 An analysis of chemokines and T-cell chemokine receptor expression in never-smokers, smokers with normal lung function, and patients with COPD showed a higher expression of the chemokine receptor CCR5 on CD8+ T cells in the blood of women smokers compared with men smokers, indicating a gender-dependent T-cell profile in COPD.44 This difference in the expression of chemokine receptors could contribute, for instance, to gender differences in disease susceptibility. CCR5 expression on CD8+ T cells has also been associated with COPD severity.45
The process of maintaining cellular homeostasis through lysosome-dependent destruction of damaged proteins, lipids, and organelles is called autophagy.46 Dysregulated autophagy, which occurs in COPD, leads to enhanced production of reactive oxidant species and contributes to airway inflammation in smokers.47 This process is important in COPD both in epithelial cells, where cigarette smoke induces aberrant autophagy and may promote cell death, and also in immune cells, where dysregulation may impair the ability of cells to clear respiratory pathogens.48
In the Karolinska COSMIC cohort, proteomic profiling of lung immune cells revealed several phagocytosis-related pathways to be more dysregulated in women, including Fc-gamma receptor (FcγR)-mediated phagocytosis, and regulation of the actin cytoskeleton, which correlated with lung function; and lysosomal pathway, which correlated with emphysema.49 Using high-resolution mass spectrometry of blood samples, increased oxidative stress in women was also demonstrated in this cohort.50 In particular, greater β-oxidation, endocannabinoid production, and purine degradation, as well as the ratios of free carnitine to medium- and long-chain acylcarnitines, were significantly increased in women relative to men.
Further, liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) of BALF samples showed upregulation of the oxidative phosphorylation pathway and downregulation of the lysosomal pathway in women with early-stage COPD vs nonsymptomatic smokers and men.51 Lysophosphatidic acid, an autotaxin product involved in phospholipid metabolism, correlated significantly with FEV1 in men, but not in women with COPD; greater increases in levels of autotaxin-regulating micro RNA in bronchoalveolar lavage cells of male COPD patients relative to female patients were also seen.50 An analysis of oxidative stress markers demonstrated significantly higher systemic lipid peroxidation levels in women than in men with COPD, which was associated with a decrease in exercise capacity.52 Hence, we see greater oxidative stress in women as compared with men, which may relate to an upregulation of protective antioxidant pathways in men.
Further quantification of tryptophan and metabolites (serotonin, kynurenine, and kynurenic acid) by LC–MS/MS in this cohort was also performed.53 In this exploratory analysis of 38 participants with COPD, 39 never-smokers and 40 smokers without COPD, major differences between tryptophan and metabolites were not seen between men and women. However, an increase in serum serotonin in women better correlated with BAL CD4+ and CD8+ T-cell counts in female but not male smokers suggesting a potential difference in inflammatory pathways as outlined above.
The mechanism for these sex differences in cellular function is still unknown. In a study examining blood and sputum samples from individuals with COPD, very few autosomal genes were found to be differentially expressed although, using a network analysis approach, significant differences in targeting patterns between men and women were seen, suggesting entirely different regulatory networks.54 However, another study examining specifically the transcriptome of circulating leukocytes before and after smoking in COPD patients and resistant smokers (with normal spirometry) identified sex-specific differences in the transcriptomic response of peripheral leukocytes, suggesting smoking itself may evoke a sex-specific phenotype.55
Structural and Physiological Differences
Changes in lung structure could also influence disease presentation.56–58 Genome-wide, single-nucleotide polymorphism (SNP)-by-sex interaction testing demonstrated that an SNP in the cadherin EGF LAG seven-pass G-type receptor 1 (CELSR1) gene was associated with COPD in women but not in men.59 CELSR1 is involved in early lung development, which could ultimately contribute to structural differences in adulthood.59 In a study of intrathoracic tracheal collapsibility in patients with COPD, women with COPD had a significantly greater degree of tracheal collapsibility than men.56 Furthermore, women with a predominant conductive airway phenotype had a significantly greater degree of collapsibility than women with a predominant emphysema phenotype. While, in this study, no relationship was observed between intrathoracic tracheal collapsibility and symptoms, a separate study on expiratory central airway collapse (ECAC) identified on CT imaging found female sex to be associated with higher prevalence of ECAC, and ECAC was associated with increased symptoms as measured by the St. George’s Respiratory Questionnaire score.60
Data from the National Emphysema Treatment Trial also suggest disease distribution and histological differences between men and women with COPD.61 In patients with severe COPD, CT images showed that women had less extensive emphysema, characterized by less peripheral involvement, and smaller hole size compared with men.61 Furthermore, women reported greater breathlessness compared with men at similar degrees of airflow obstruction and emphysema severity.61 Interestingly, an analysis of a subset of National Lung Screening Trial CT scans also showed that men had more emphysema than women at all stages of COPD severity.62 Similarly, quantitative analysis of high-resolution CT images from a patient population of smokers with relatively early-onset severe COPD (International COPD Genetics Network [ICGN]) also demonstrated that men had more severe emphysema, even after adjusting for lung function and smoking history.63 In a small Chinese study, again men had greater extent of emphysema than women.64 In COPDGene, men were also found to have greater overall percent emphysema, except a subset of women with early-onset COPD who had similar emphysema to men but lesser smoking history than men.65 In another analysis, multidetector CT scans of heavy smokers recruited for a lung cancer screening project, showed that women had a less extensive emphysema phenotype in each pulmonary lobe than men – characterized by smaller areas and slightly less concentrated in the core of the lung.66 Overall, these data suggest that while women with COPD on average may have less emphysema, emphysema distribution may differ from men. Further, there appear to be subgroups of women who are still particularly susceptible to parenchymal destruction.
With respect to airways disease, among the generally severe, emphysematous patient population analyzed in the NETT, histological analyses of resected tissue revealed that women had smaller airway lumens with thicker airway walls than men.61 However, quantitative analysis of high-resolution CT images from the ICGN patient population failed to detect a gender difference in airway wall thickness63 as did the small Chinese study referenced above.64 We must also consider whether such differences relate to female gender specifically as opposed to just an impact of COPD in women. In a separate retrospective study of individuals with COPD, larger wall area percent but smaller lumen diameter and wall thickness were seen regardless of smoking status.67 Further, in an analysis of non-smokers without COPD recruited into the COPDGene study, greater wall area percentage was still seen in women as compared with men.68 Hence from these data, it is difficult to know to what extent airway alterations relate to gender or a combination of gender and COPD pathobiology. However, it is conceivable that pathologically smaller airway lumens in women relative to men could contribute to increased symptoms.
While sex-specific differences in pulmonary function and exercise performance have previously been established in normal individuals,69 results of a cross-sectional analysis showed that men and women with COPD, even at equivalent levels of pulmonary dysfunction, differed in decline in functional aerobic capacity.69 While men showed progressive loss of body weight, exercise ability, oxygen pulse, and maximum exercise ventilation with mild pulmonary dysfunction, women did not lose weight and maintained usual exercise ability and oxygen pulse until progression to moderate or severe disease.69 While the exact underlying mechanisms responsible for the decline in functional aerobic capacity are unknown, the difference in functional capacity may be related to a decrease in oxygen pulse – an indicator of cardiac stroke volume – occurring earlier in the natural history of the disease in men as compared with women.69 At the same time, however, women may still demonstrate more breathlessness for a given level of exercise. A study examining cardiopulmonary exercise testing showed that women had a greater intensity of dyspnea during a given ventilation and work rate.57 Differences in body composition between the sexes may play a role in the differences in clinical presentation. Results of a recent study of women participating in the UK Biobank showed that reproductive health indicators such as late menarche (>15 years of age), early menopause (<47 years of age), parity >3, history of polycystic ovary syndrome, hormone replacement therapy use, or hysterectomy were associated with a greater risk of COPD-related hospitalization/death whereas oral contraception use was associated with lower risk of COPD-related hospitalization/death.70
Discussion
Gender differences in the clinical presentation and prognosis for COPD have been previously described.25,71–74 For example, women have more severe COPD symptoms than men,25 despite less smoking exposure history.61,71 Furthermore, women have a greater risk of exacerbations (after adjusting for smoking)24,25 and, COPD-related hospitalization and death compared with men.75,76 Other factors, such as comorbidities and adherence to treatment may also differ between men and women and should be considered.72,74 A goal of this literature review was to investigate biological factors potentially underlying these differences. Overall, we found the number of such studies to be limited. However, the studies we found support sex differences in cell function and regulation as well as structural and physiological differences, which may contribute to the differences noted in clinical phenotype.
A common theme identified across several studies was an increase in inflammatory markers in women with COPD, which may relate to differences in body composition. In particular, leptin levels correlated with plasma CRP concentration in women with COPD, but not in men.30 Leptin has previously been associated with emphysema36,77 and low lung function.77 Leptin is known to be elevated even among healthy women compared with healthy men and may be driven by differences in fat distribution.33,34 Therefore, it is possible that elevated leptin levels in women with COPD contribute to sex differences in disease pathology and clinical course. Gender differences in body composition may also increase other inflammatory mediators.38 In the ECLIPSE study, higher subcutaneous adipose tissue area was associated with greater CRP and fibrinogen levels in women.38 In another study, IL-16 was associated with BMI in women with COPD but not in men.39 Previously, IL-16 has been associated with asthma78 and smokers with chronic bronchitis79 and, hence, could contribute or reflect phenotypic differences that have been observed in women. The SAPALDIA study also demonstrated that weight gain, increased CRP, and rapid FEV1 decline were uniquely associated in women but not in men,42 again suggesting that body composition may mediate the relationship between gender and increased inflammation in COPD.
Dysregulation of the immune response was also noted in several studies as a possible contributor to gender differences in COPD. For example, gender differences in the expression of CCR5 on CD8+T cells may cause variations in T-cell recruitment to the lungs,44 contributing to the inflammatory process.80 Dysregulation of autophagy was also found in women with COPD.49 In the Karolinska COSMIC cohort,49 proteomic profiling of lung immune cells revealed several phagocytosis-related pathways to be more dysregulated in women and correlated with lung function and emphysema.49 Correspondingly, increased oxidative stress in women with COPD compared with men were also noted.50–52 Oxidative stress pathways are believed to contribute to COPD development81 as well as exacerbations.82 While the mechanism for these differences is not completely understood, smoking-induced sex-specific differences in the transcriptomic response of peripheral leukocytes suggest smoking may induce a sex-specific phenotype.55
Finally, histological, physiological, and structural differences associated with the lung were observed between men and women with COPD.56,61,67 In few studies, women had smaller airway lumen, thicker airway walls, and less extensive emphysema than men.61,62,65 A predominance of emphysema could partially account for the reduction in maximum exercise ventilation reported in men with COPD.69 Moreover, several reproductive health indicators were associated with a greater risk of COPD-related hospitalization/death suggesting that female reproductive hormones may play a role in the pathogenesis of COPD.70 Additional studies are needed to improve our understanding on how these differences contribute to the overall clinical manifestations of COPD and ultimately treatment.
The ultimate question is whether these biological differences result in differential response to therapy. Unfortunately, data to date regarding differential therapeutic responses are inconsistent.83–86 A subgroup analysis of data from the Understanding the Potential Long-term Impact of Tiotropium (UPLIFT®) study reported that tiotropium resulted in similar improvements in lung function, exacerbations, and health status in men and women.83 Similarly, in the TRial of Inhaled STeroids ANd long-acting β2 agonists (TRISTAN) study, salmeterol/fluticasone combination therapy produced significant improvements compared with placebo in lung function, exacerbation, and health-related quality of life in men and women.85 In a post hoc analysis of the FLAME study, indacaterol/glycopyrronium reduced exacerbations and improved lung functions in men and women.86 However, in an analysis of pooled data from 6108 patients with moderate to very severe COPD who participated in 6 studies of the IGNITE program, while treatment with indacaterol/glycopyrronium showed similar improvements in lung function, improvements in health status, dyspnea, rescue medication use, and symptoms were generally greater in women vs men.84 One possibility is that similar increases in airway diameter may have greater impact in women where airway diameters are already smaller to begin with. Therefore, given the potential biologic gender differences we have identified in this review as they relate to biologic pathways, further studies examining therapeutic differences in men and women with COPD are clearly warranted.
We acknowledge that this analysis has several limitations. Although our search was extensive, men were the sole focus of almost one-third of the screened articles. Therefore, only a limited number of articles focusing on COPD in women could be included. In addition, statistical analysis (ie, meta-analysis) of the data was not feasible due to the inclusion of a diverse range of studies with differing patient populations and outcome measures.
Conclusion
Results of this systematic review indicate that sex may influence cell function and inflammatory pathways as well as histological, structural, and physiological differences that may contribute to differences in the clinical expression of COPD observed between men and women. However, further studies are needed to link these biological mechanisms with clinical observations. An improved understanding of these underlying differences is important for improved diagnosis, treatment, and monitoring of COPD in women.
Acknowledgments
The author meets the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The author received no direct compensation related to the development of this manuscript. Writing, editorial support, and formatting assistance was provided by Suchita Nath-Sain, PhD, Maribeth Bogush, MCI, PhD, and Frances Gambling, of Cactus Life Sciences (part of Cactus Communications), which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author Contributions
Dr Han contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agrees to be accountable for all aspects of the work.
Disclosure
MeiLan K. Han reports consulting fees from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Merck, and Novartis. She also reports research support from Novartis and Sunovion. Boehringer Ingelheim also supported conduction of the systematic search.
References
- 1.Boudewijns EA, Babu GR, Salvi S, Sheikh A, van Schayck OC. Chronic obstructive pulmonary disease: a disease of old age? J Glob Health. 2018;8(2):020306. doi: 10.7189/jogh.08.020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aryal S, Diaz-Guzman E, Mannino DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:1145–1154. doi: 10.2147/COPD.S54476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff EW, Schermer TR, Bor H, Brown P, van Weel C, van den Bosch WJ. Trends in COPD prevalence and exacerbation rates in Dutch primary care. Br J Gen Pract. 2009;59(569):927–933. doi: 10.3399/bjgp09X473079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 5.Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010;170(6):560–565. doi: 10.1001/archinternmed.2010.17 [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ntritsos G, Franek J, Belbasis L, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:1507–1514. doi: 10.2147/COPD.S146390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Durme YMTA, Verhamme KMC, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–377. doi: 10.1378/chest.08-0684 [DOI] [PubMed] [Google Scholar]
- 9.Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi: 10.1164/rccm.201011-1928OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States, 2000–2014. NCHS Data Brief. 2016;(256):1–8. Hyattsville, MD: NCHS Data Brief. [PubMed] [Google Scholar]
- 12.Sana A, Somda SMA, Meda N, Bouland C. Chronic obstructive pulmonary disease associated with biomass fuel use in women: a systematic review and meta-analysis. BMJ Open Respir Res. 2018;5(1):e000246. doi: 10.1136/bmjresp-2017-000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aryal S, Diaz-Guzman E, Mannino DM. COPD and gender differences: an update. Transl Res. 2013;162(4):208–218. doi: 10.1016/j.trsl.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 16.Dai MY, Qiao JP, Xu YH, Fei GH. Respiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD assessment test. Int J Chron Obstruct Pulmon Dis. 2015;10:2257–2263. doi: 10.2147/COPD.S92160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. doi: 10.1378/chest.10-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez Varela MV, Montes de Oca M, Halbert RJ, et al. Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J. 2010;36(5):1034–1041. doi: 10.1183/09031936.00165409 [DOI] [PubMed] [Google Scholar]
- 19.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(6):1110–1116. doi: 10.1016/j.rmed.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 20.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–2158. doi: 10.1164/ajrccm.162.6.2003112 [DOI] [PubMed] [Google Scholar]
- 21.Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7:52. doi: 10.1186/1465-9921-7-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral AFS, Strachan DP, Burney PGJ, Jarvis DL. Female smokers are at greater risk of airflow obstruction than male smokers. UK biobank. Am J Respir Crit Care Med. 2017;195(9):1226–1235. doi: 10.1164/rccm.201608-1545OC [DOI] [PubMed] [Google Scholar]
- 23.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi: 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celli B, Vestbo J, Jenkins CR, et al. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am J Respir Crit Care Med. 2011;183(3):317–322. doi: 10.1164/rccm.201004-0665OC [DOI] [PubMed] [Google Scholar]
- 25.DeMeo DL, Ramagopalan S, Kavati A, et al. Women manifest more severe COPD symptoms across the life course. Int J Chron Obstruct Pulmon Dis. 2018;13:3021–3029. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 27.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. doi: 10.1016/j.ccm.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 28.Lundström SL, Balgoma D, Wheelock ÅM, Haeggström JZ, Dahlén SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Curr Pharm Biotechnol. 2011;12(7):1026–1052. doi: 10.2174/138920111795909087 [DOI] [PubMed] [Google Scholar]
- 29.Balgoma D, Yang M, Sjödin M, et al. Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. Eur Respir J. 2016;47(6):1645–1656. doi: 10.1183/13993003.01080-2015 [DOI] [PubMed] [Google Scholar]
- 30.Breyer MK, Rutten EP, Vernooy JH, et al. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med. 2011;105(7):1046–1053. doi: 10.1016/j.rmed.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie. 2012;94(10):2180–2189. doi: 10.1016/j.biochi.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman JM. Leptin and the regulation of body weight. Keio J Med. 2011;60(1):1–9. doi: 10.2302/kjm.60.1 [DOI] [PubMed] [Google Scholar]
- 33.Kennedy A, Gettys TW, Watson P, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82(4):1293–1300. doi: 10.1210/jcem.82.4.3859 [DOI] [PubMed] [Google Scholar]
- 34.Lönnqvist F, Wennlund A, Arner P. Relationship between circulating leptin and peripheral fat distribution in obese subjects. Int J Obes Relat Metab Disord. 1997;21(4):255–260. doi: 10.1038/sj.ijo.0800394 [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Li D, Wang H, Pang C, Wu Y, Wen F. Gender difference in plasma fatty-acid-binding protein 4 levels in patients with chronic obstructive pulmonary disease. Biosci Rep. 2016;36(1):e00302. doi: 10.1042/BSR20150281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M, Makita H, Östling J, et al. Lower leptin/adiponectin ratio and risk of rapid lung function decline in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(10):1511–1519. doi: 10.1513/AnnalsATS.201408-351OC [DOI] [PubMed] [Google Scholar]
- 37.Krommidas G, Kostikas K, Papatheodorou G, et al. Plasma leptin and adiponectin in COPD exacerbations: associations with inflammatory biomarkers. Respir Med. 2010;104(1):40–46. doi: 10.1016/j.rmed.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 38.Diaz AA, Zhou L, Young TP, et al. Chest CT measures of muscle and adipose tissue in COPD: gender-based differences in content and in relationships with blood biomarkers. Acad Radiol. 2014;21(10):1255–1261. doi: 10.1016/j.acra.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Torres JP, Casanova C, Pinto-Plata V, et al. Gender differences in plasma biomarker levels in a cohort of COPD patients: a pilot study. PLoS One. 2011;6(1):e16021. doi: 10.1371/journal.pone.0016021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson S, Nordenson A, Glader P, Yoshihara S, Lindén A, Slinde F. A gender difference in circulating neutrophils in malnourished patients with COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:83–88. doi: 10.2147/COPD.S15351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharanya A, Ciano M, Withana S, Kemp PR, Polkey MI, Sathyapala SA. Sex differences in COPD-related quadriceps muscle dysfunction and fibre abnormalities. Chron Respir Dis. 2019;16:1479973119843650. doi: 10.1177/1479973119843650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bridevaux PO, Gerbase MW, Schindler C, et al. Sex-specific effect of body weight gain on systemic inflammation in subjects with COPD: results from the SAPALDIA cohort study 2. Eur Respir J. 2009;34(2):332–339. doi: 10.1183/09031936.00162608 [DOI] [PubMed] [Google Scholar]
- 43.Ólafsdóttir IS, Gíslason T, Thjóđleifsson B, et al. Gender differences in the association between C-reactive protein, lung function impairment, and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):635–642. [PMC free article] [PubMed] [Google Scholar]
- 44.Forsslund H, Yang M, Mikko M, et al. Gender differences in the T-cell profiles of the airways in COPD patients associated with clinical phenotypes. Int J Chron Obstruct Pulmon Dis. 2017;12:35–48. doi: 10.2147/COPD.S113625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol. 2007;171(3):767–776. doi: 10.2353/ajpath.2007.061177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15(7):713–720. doi: 10.1038/ncb2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monick MM, Powers LS, Walters K, et al. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185(9):5425–5435. doi: 10.4049/jimmunol.1001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14(2):221–232. doi: 10.1080/15548627.2017.1389823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M, Kohler M, Heyder T, et al. Proteomic profiling of lung immune cells reveals dysregulation of phagocytotic pathways in female-dominated molecular COPD phenotype. Respir Res. 2018;19(1):39. doi: 10.1186/s12931-017-0699-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naz S, Kolmert J, Yang M, et al. Metabolomics analysis identifies sex-associated metabotypes of oxidative stress and the autotaxin–lysoPA axis in COPD. Eur Respir J. 2017;49(6):1602322. doi: 10.1183/13993003.02322-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohler M, Sandberg A, Kjellqvist S, et al. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):743–751. doi: 10.1016/j.jaci.2012.09.024 [DOI] [PubMed] [Google Scholar]
- 52.Maury J, Gouzi F, De Rigal P, et al. Heterogeneity of systemic oxidative stress profiles in COPD: a potential role of gender. Oxid Med Cell Longev. 2015;2015:201843. doi: 10.1155/2015/201843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naz S, Bhat M, Stahl S, et al. Dysregulation of the tryptophan pathway evidences gender differences in COPD. Metabolites. 2019;9(10):212. doi: 10.3390/metabo9100212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glass K, Quackenbush J, Silverman EK, et al. Sexually-dimorphic targeting of functionally-related genes in COPD. BMC Syst Biol. 2014;8(1):118. doi: 10.1186/s12918-014-0118-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faner R, Gonzalez N, Cruz T, Kalko SG, Agustí A. Systemic inflammatory response to smoking in chronic obstructive pulmonary disease: evidence of a gender effect. PLoS One. 2014;9(5):e97491. doi: 10.1371/journal.pone.0097491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camiciottoli G, Diciotti S, Bigazzi F, et al. Is intrathoracic tracheal collapsibility correlated to clinical phenotypes and sex in patients with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:843–852. doi: 10.2147/COPD.S80558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O’Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: physiological mechanisms. Respir Physiol Neurobiol. 2011;177(3):218–227. doi: 10.1016/j.resp.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 58.Smith BM, Traboulsi H, Austin JHM, et al. Human airway branch variation and chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2018;115(5):E974–E981. doi: 10.1073/pnas.1715564115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardin M, Cho MH, Sharma S, et al. Sex-based genetic association study identifies CELSR1 as a possible chronic obstructive pulmonary disease risk locus among women. Am J Respir Cell Mol Biol. 2017;56(3):332–341. doi: 10.1165/rcmb.2016-0172OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatt SP, Terry NL, Nath H, et al. Association between expiratory central airway collapse and respiratory outcomes among smokers. JAMA. 2016;315(5):498–505. doi: 10.1001/jama.2015.19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–252. doi: 10.1164/rccm.200606-828OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132(2):464–470. doi: 10.1378/chest.07-0863 [DOI] [PubMed] [Google Scholar]
- 63.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480–1488. doi: 10.1378/chest.09-0676 [DOI] [PubMed] [Google Scholar]
- 64.Gu S, Deng X, Li Q, Sun X, Xu J, Li H. Gender differences of chronic obstructive pulmonary disease associated with manifestations on HRCT. Clin Respir J. 2017;11(1):28–35. doi: 10.1111/crj.12297 [DOI] [PubMed] [Google Scholar]
- 65.Hardin M, Foreman M, Dransfield MT, et al. Sex-specific features of emphysema among current and former smokers with COPD. Eur Respir J. 2016;47(1):104–112. doi: 10.1183/13993003.00996-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sverzellati N, Calabro E, Randi G, et al. Sex differences in emphysema phenotype in smokers without airflow obstruction. Eur Respir J. 2009;33(6):1320–1328. doi: 10.1183/09031936.00109808 [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Dai YL, Yu N, Guo YM. Sex-related differences in bronchial parameters and pulmonary function test results in patients with chronic obstructive pulmonary disease based on three-dimensional quantitative computed tomography. J Int Med Res. 2018;46(1):135–142. doi: 10.1177/0300060517721309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zach JA, Newell JDJ, Schroeder J, et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47(10):596–602. doi: 10.1097/RLI.0b013e318262292e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter R, Nicotra B, Huber G. Differing effects of airway obstruction on physical work capacity and ventilation in men and women with COPD. Chest. 1994;106(6):1730–1739. doi: 10.1378/chest.106.6.1730 [DOI] [PubMed] [Google Scholar]
- 70.Tang R, Fraser A, Magnus MC. Female reproductive history in relation to chronic obstructive pulmonary disease and lung function in UK biobank: a prospective population-based cohort study. BMJ Open. 2019;9(10):e030318. doi: 10.1136/bmjopen-2019-030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeMeo DL. The yin and yang of COPD: sex/gender differences in the National Emphysema Treatment Trial. Am J Respir Crit Care Med. 2007;176(3):222–223. doi: 10.1164/rccm.200704-558ED [DOI] [PubMed] [Google Scholar]
- 72.Jenkins CR, Chapman KR, Donohue JF, Roche N, Tsiligianni I, Han MK. Improving the management of COPD in women. Chest. 2017;151(3):686–696. doi: 10.1016/j.chest.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 73.Ohar J, Fromer L, Donohue JF. Reconsidering sex-based stereotypes of COPD. Prim Care Respir J. 2011;20(4):370–378. doi: 10.4104/pcrj.2011.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicolini A, Barbagelata E, Tagliabue E, Colombo D, Monacelli F, Braido F. Gender differences in chronic obstructive pulmonary diseases: a narrative review. Panminerva Med. 2018;60(4):192‒199. doi: 10.23736/S0031-0808.18.03463-8 [DOI] [PubMed] [Google Scholar]
- 75.Prescott E, Bjerg AM, Andersen PK, Lange P, Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J. 1997;10(4):822–827. [PubMed] [Google Scholar]
- 76.Goel K, Bailey M, Borgstrom M, et al. Trends in chronic obstructive pulmonary disease hospitalization and in-hospital deaths in the United States by sex: 2005 to 2014. Ann Am Thorac Soc. 2019;16(3):391–393. doi: 10.1513/AnnalsATS.201807-488RL [DOI] [PubMed] [Google Scholar]
- 77.Oh YM, Jeong BH, Woo SY, et al. Association of plasma adipokines with chronic obstructive pulmonary disease severity and progression. Ann Am Thorac Soc. 2015;12(7):1005–1012. doi: 10.1513/AnnalsATS.201501-005OC [DOI] [PubMed] [Google Scholar]
- 78.Burkart KM, Barton SJ, Holloway JW, et al. Association of asthma with a functional promoter polymorphism in the IL16 gene. J Allergy Clin Immunol. 2006;117(1):86–91. doi: 10.1016/j.jaci.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 79.Andersson A, Qvarfordt I, Laan M, et al. Impact of tobacco smoke on interleukin-16 protein in human airways, lymphoid tissue and T lymphocytes. Clin Exp Immunol. 2004;138(1):75–82. doi: 10.1111/j.1365-2249.2004.02580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol. 2007;178(12):8090–8096. doi: 10.4049/jimmunol.178.12.8090 [DOI] [PubMed] [Google Scholar]
- 81.Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018:5730395. doi: 10.1155/2018/5730395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Global Initiative for Chronic Obstructive Lung Disease. GOLD; 2020. Available from: https://goldcopd.org. Accessed December23, 2019.
- 83.Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: results of the 4-year UPLIFT trial. Respir Med. 2010;104(10):1495–1504. doi: 10.1016/j.rmed.2010.03.033 [DOI] [PubMed] [Google Scholar]
- 84.Tsiligianni I, Mezzi K, Fucile S, et al. Response to indacaterol/glycopyrronium (IND/GLY) by sex in patients with COPD: a pooled analysis from the IGNITE program. Copd. 2017;14(4):375–381. doi: 10.1080/15412555.2017.1324837 [DOI] [PubMed] [Google Scholar]
- 85.Vestbo J, Soriano JB, Anderson JA, Calverley P, Pauwels R, Jones P. Gender does not influence the response to the combination of salmeterol and fluticasone propionate in COPD. Respir Med. 2004;98(11):1045–1050. doi: 10.1016/j.rmed.2004.03.017 [DOI] [PubMed] [Google Scholar]
- 86.Wedzicha JA, Singh D, Tsiligianni I, et al. Treatment response to indacaterol/glycopyrronium versus salmeterol/fluticasone in exacerbating COPD patients by gender: a post-hoc analysis in the FLAME study. Respir Res. 2019;20(1):4. doi: 10.1186/s12931-019-0972-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease. GOLD; 2020. Available from: https://goldcopd.org. Accessed December23, 2019.