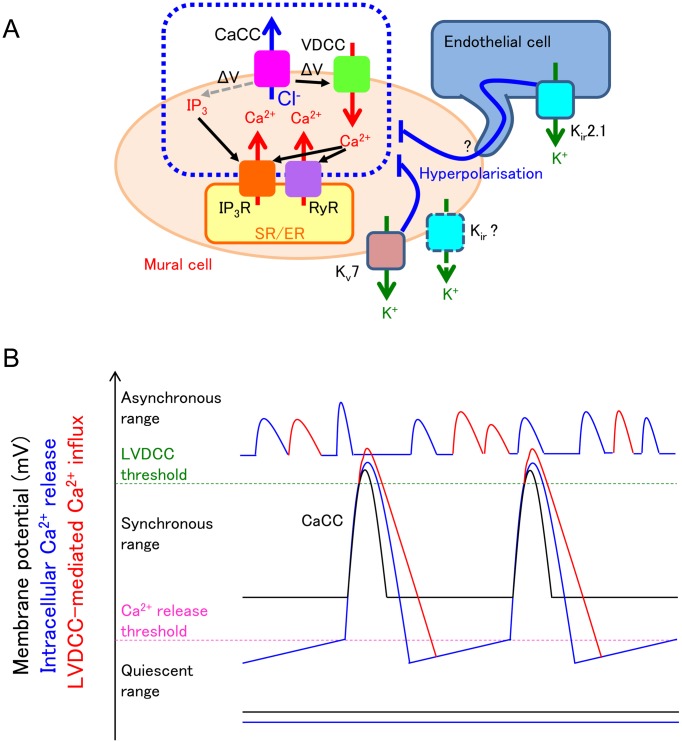
Fig. 8.
Fundamental roles of the membrane potential in maintaining the synchrony of spontaneous Ca2+ transients.
A: Kv7 voltage-dependent K+ (Kv7) channels and inward rectifier K+ (Kir) channels are open under resting condition to hyperpolarise the mural cells. This hyperpolarisation inhibits voltage-dependent IP3 production and subsequent IP3 receptor (IP3R)-mediated Ca2+ release and also decreases voltage-dependent Ca2+ channel (VDCC)-mediated Ca2+ influx and subsequent Ca2+-induced Ca2+ release via ryanodine receptors (RyR) and/or IP3R. A resultant decrease in the frequency of spontaneous Ca2+ release from the sarcoendoplasmic reticulum (SR/ER) ensures the enough Ca2+ refilling in the SR/ER; thus, each spontaneous Ca2+ release from SR/ER is large enough to induce Ca2+-activated Cl- channel (CaCC)-mediated depolarisation that leads to recruitment/activation of more store Ca2+ release events and their synchronisation within the network of mural cells. B: When the resting membrane potential of mural cells are within the ‘synchronous range’, cyclical spontaneous Ca2+ release from SR/ER (blue line) opens CaCCs to induce cyclical spontaneous depolarisation (black line). The depolarisation causes L-type voltage-dependent Ca2+ channel (LVDCC)-mediated Ca2+ influx (red line). These spontaneous activities spread to neighbouring mural cells via gap junctions. When the resting membrane potential of mural cells are within the ‘quiescent range’, voltage-dependent IP3 production is suppressed. Thus, cyclical spontaneous Ca2+ release from SR/ER is now not generated (blue flat line), and membrane potential change is not detected (black flat line). When the resting membrane potential is higher than the threshold of LVDCC, i.e., within the ‘asynchronous range’, both cyclical spontaneous Ca2+ release from SR/ER (blue line) and LVDCC-mediated Ca2+ influx (red line) cause high frequency spontaneous Ca2+ transients that are generated independently among mural cells.