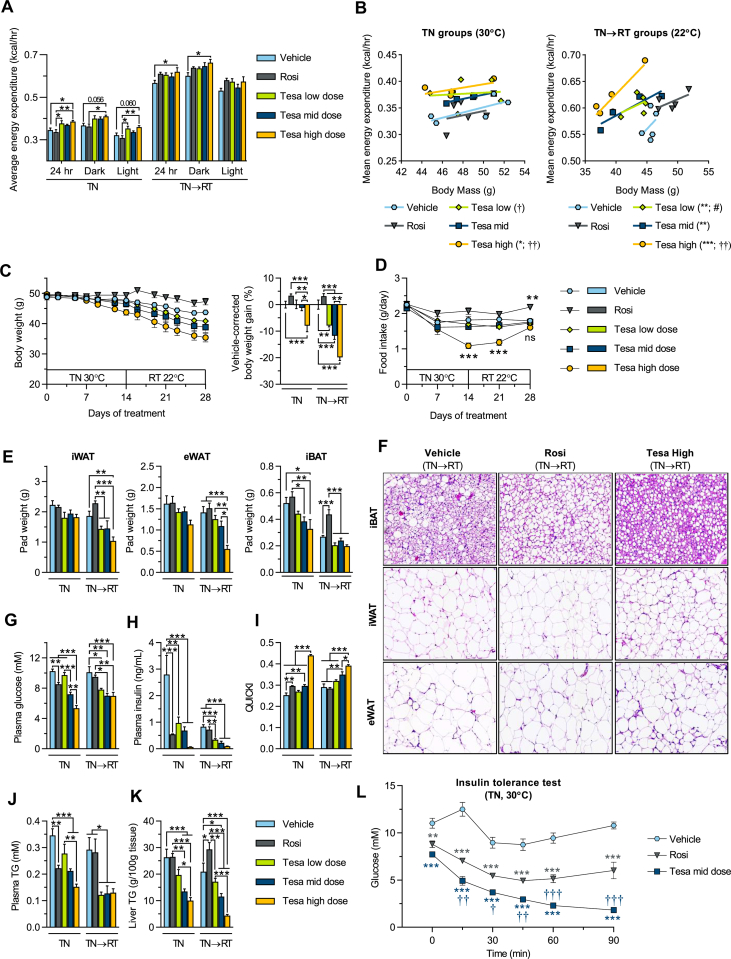
Figure 4.
Tesaglitazar increases energy expenditure, reduces body weight, improves metabolic control and ameliorates high fat diet-induced hepatic steatosis in obese mice. DIO male mice were used in conditions and treatments as described in Figure 3A. (a) Average energy expenditure for the full day (24 h), dark and light periods at TN (30 °C, TN group) and RT (22 °C, TN→RT group). (b) Regression plots of average energy expenditure vs. body mass. (c) Body weight over treatment time and vehicle-corrected body weight gain. (d) Food intake over treatment time. (e) Terminal iWAT, eWAT and iBAT fat pad weights. (f) Hematoxylin & Eosin staining in iBAT, iWAT and eWAT of vehicle, Rosi and Tesa high dose treated TN→RT mice. (g) Plasma glucose, (h) plasma insulin (i) QUICKI insulin sensitivity indexes, (j) plasma TG, (k) liver TG content and (l) plasma glucose levels following an insulin tolerance test (ITT, 0.75 U/kg) in vehicle, Rosi and Tesa mid dose treated TN mice. Data presented as mean ± SEM (n = 4–5/group): ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs. indicated groups in a, c, e and g–k: ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 vs. vehicle, †P < 0.05; ††P < 0.01; †††P < 0.001 vs. rosiglitazone and #P < 0.05 vs. Tesa high dose in b, d and l by one-way (a, c, e and g-k) or two-way (d and l) ANOVA or ANCOVA with body mass as covariate (b), all with Tukey's multiple comparisons test.