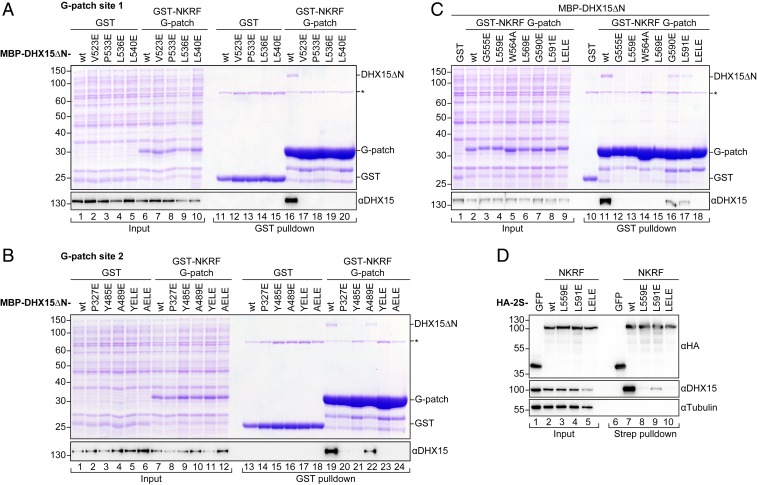
Fig. 3.
Mutations in the two binding sites have different effects on complex formation. (A–C) Coomassie-stained gels of copurification assays of MBP–DHX15ΔN and GST–NKRF G-patch coexpressed in E. coli. Input (0.03%) and eluates (4.4%) were loaded. GST served as a control. DHX15 expression was verified by Western blotting using an antibody against DHX15. The asterisk marks an unspecific band that copurifies with GST from E. coli lysates in all conditions. DHX15 mutations in G-patch sites 1 and 2 are shown in A and B, respectively. YELE corresponds to double mutant Y485E, L536E, while AELE stands for A489E, L540E. Mutations in the G-patch motif are shown in C. (D) Western blots of coprecipitation assays from HEK293T cells overexpressing HA-2S-NKRF or HA-2S-GFP as a control. Samples were treated with RNase A and tested for copurification of endogenous DHX15. Tubulin served as a loading control. For anti-HA blots, 0.75% of input and 5% of eluates were loaded, while 0.25% of input and 15% of eluates were loaded for anti-DHX15 and anti–α-tubulin blots.