Summary
The coordination of synaptic vesicle exocytosis and endocytosis supports neurotransmitter release from presynaptic terminals. Although inositol pyrophosphates, such as 5-diphosphoinositol pentakisphosphate (5-IP7), are versatile signaling metabolites in many biological events, physiological actions of 5-IP7 on synaptic membrane vesicle trafficking remain unclear. Here, we investigated the role of 5-IP7 in synaptic transmission in hippocampal brain slices from inositol hexakisphosphate kinase 1 (Ip6k1)-knockout mice. We found that presynaptic release probability was significantly increased in Ip6k1-knockout neurons, implying enhanced activity-dependent synaptic vesicle exocytosis. Expression of wild-type but not catalytically inactive IP6K1 in the Ip6k1-knockout hippocampus restored the altered presynaptic release probability. Moreover, Ip6k1-knockout neurons were insensitive to folimycin, a vacuolar ATPase inhibitor, and dynasore, a dynamin inhibitor, suggesting marked impairment in synaptic endocytosis during exocytosis. Our findings collectively establish that IP6K1 and its product, 5-IP7, act as key physiological determinants for inhibition of presynaptic vesicle exocytosis and stimulation of endocytosis at central synapses.
Subject Areas: Neurogenetics, Neuroscience, Cell Biology
Graphical Abstract

Highlights
-
•
Excitatory synaptic vesicle release is increased in Ip6k1-KO hippocampal neurons
-
•
Wild-type IP6K1 restores presynaptic functions and plasticity of KO neurons
-
•
Catalytically inactive mutant IP6K1 fails to rescue defective presynaptic release
-
•
Synaptic endocytosis during exocytosis is severely impaired in Ip6k1-KO neurons
Neurogenetics; Neuroscience; Cell Biology
Introduction
Inositol phosphates (IPs), found in organisms ranging from yeast to mammals, act as molecular messengers that mediate diverse cellular functions, including growth and metabolism (Chakraborty et al., 2011, Hatch and York, 2010, Shears, 2015, Wilson et al., 2013). Inositol 1,4,5-trisphosphate (IP3), perhaps the best-known IP metabolite, triggers increases in cytosolic Ca2+ levels through direct binding and activation of IP3-gated Ca2+ channels in the ER(Irvine and Schell, 2001). The identification of IP kinases and phosphatases led to the discovery of higher inositol polyphosphates containing multiple phosphates, such as inositol hexakisphosphate (IP6) (Seeds et al., 2007). In mammals, the most extensively characterized inositol pyrophosphate is 5-diphosphoinositol pentakisphosphate (5-PP-[1,2,3,4,6]IP5), designated 5-IP7, which is synthesized by phosphorylation of fully phosphorylated six-carbon inositol IP6 at position 5 (Chakraborty et al., 2011, Park et al., 2018).
The biosynthesis of 5-IP7 is catalyzed by a family of three inositol hexakisphosphate kinases (IP6Ks) (Saiardi et al., 1999). Among these, IP6K1 is highly expressed in the brain (Saiardi et al., 1999); however, its neuronal functions remain poorly understood. As shown in non-neuronal cells (e.g., hepatocytes), the IP6K1 product, 5-IP7, inhibits brain Akt activity (Chakraborty et al., 2014). Moreover, IP6K1 can directly bind and stimulate glycogen synthase kinase 3 (GSK3) in a non-catalytic manner (Chakraborty et al., 2014). Ip6k1-null mice exhibit reduced GSK3 activity and thus manifest behavioral defects such as reduced locomotive responsiveness to amphetamine as well as impaired social interactions (Chakraborty et al., 2014). It was also suggested that IP6K1 catalytic activity controls α-actinin and focal adhesion kinase, based on the observation that deletion of IP6K1 leads to retarded neuronal migration in the cerebral cortex (Fu et al., 2017).
Synaptic vesicle cycling is a fundamental process in cell-to-cell communication that underlies virtually all neural activities, as exemplified by neurotransmitter release (Schweizer and Ryan, 2006, Südhof, 2004). Since the early 1990s, IPs have been highlighted for their role as endogenous signaling metabolites that influence synaptic vesicle cycling (Llinás et al., 1994, Yang et al., 2012). 5-IP7 was shown to exert potent inhibitory activity toward synaptic vesicle exocytosis through direct inhibition of synaptotagmin 1 (Syt1), a Ca2+ sensor essential for synaptic membrane fusion (Lee et al., 2016). In PC12 cells, increasing 5-IP7 levels by overexpressing IP6K1 suppresses depolarization-induced neurotransmitter release, whereas decreasing 5-IP7 concentrations by IP6K1 knockdown leads to enhanced exocytosis. IP6K1 knockdown in cultured hippocampal neurons augments action-potential-driven synaptic vesicle exocytosis (Lee et al., 2016). By contrast, IP6K1 was previously found to non-catalytically stimulate dopamine release in PC12 cells through direct protein-protein interaction with GRAB, a guanine nucleotide exchange factor for Rab3A (Luo et al., 2001). In non-neuronal cells, IP6K1 and 5-IP7 have also been implicated in the fine control of endocytosis. Budding yeast devoid of KCS1, the S. cerevisiae homolog of mammalian IP6K1, exhibits defective endocytic membrane trafficking (Saiardi et al., 2002). Nonetheless, physiological neuronal functions of 5-IP7 metabolism in the regulation of synaptic vesicle cycling have remained largely elusive.
In this study, we electrophysiologically investigated synaptic vesicle cycling in Ip6k1-knockout (KO) mice. We found that the loss of IP6K1 enhanced presynaptic release probability and deregulated synaptic vesicle endocytosis at active hippocampal synapses, thereby establishing physiological roles of IP6K1 and inositol pyrophosphates in the fine control of synaptic vesicle exocytosis as well as endocytic recycling.
Results
Generation of IP6K1-KO Mice
To investigate physiological roles of IP6K1 in the control of synaptic vesicle trafficking, we employed the CRISPR/Cas9 system to generate mice with a targeted deletion of IP6K1. To ensure complete disruption of functional proteins, we chose target sites near the translational start ATG codon in exon 2 of mouse Ip6k1 (Figure 1A). The gRNA-Cas9 system induced the expected mutation in the targeting site, and the mutated allele included a 73-bp deletion that resulted in a frameshift and premature termination (Figure 1B). Immunoblotting of brain extracts using IP6K1-specific antibodies confirmed the absence of IP6K1 protein in homozygous Ip6k1 exon 2-deleted (IP6K1-KO) mice (Figure 1C). We next examined whether IP6K1 catalytic activity was lost in IP6K1-KO mice by measuring IP7 levels from IP6K1-KO and wild-type (WT) mice. Similar to a previous report by Chakraborty et al. using IP6K1-KO mice harboring a deletion of exon 6 corresponding to the kinase domain (Chakraborty et al., 2010), we found that IP7 levels in primary hippocampal neurons (Figure 1D) and hepatocytes (Figure S1A) from our IP6K1-KO mice were markedly reduced (∼60% and ∼50%, respectively) and insulin-stimulated Akt activation was enhanced in IP6K1-KO hepatocytes (Figure S1B), confirming the successful generation of IP6K1-KO mice through targeting of the ATG start codon. Using Nissl staining to analyze brain architecture, we found that brain structure was normal in both 5-week-old IP6K1-KO mice (Figure 1E) and 8-week-old IP6K1-KO mice (Figure S2A). An analysis of the anatomy of the hippocampus, the primary target of our electrophysiological analyses, showed that hippocampal formation was normal in young (5-week-old, Figure 1F) and adult (8-week-old, Figure S2B) IP6K1-KO mouse brains, confirming that loss of IP6K1 does not affect hippocampal development. Expression of two other IP6K isoforms (IP6K2 and IP6K3) was not altered by the loss of IP6K1 (Figure S3). IP6K1 was detected in synaptic fractions, including crude synaptosomes, synaptic membranes, and synaptic vesicle fractions of the mouse brain (Figure S4), providing evidence for the synaptic presence of IP6K1. We further checked that IP6K1-KO brain expresses normal amounts of synaptic proteins, including synaptotagmin, dynamin, presynaptic vesicular glutamate transporters, AP2, v-ATPase, phosphatidylinositol 4-phosphate 5-kinase (Figure S5).
Figure 1.
Generation and Characterization of IP6K1-KO Mice
(A) Schematic depiction of the strategy for Ip6k1 gene disruption. Sequences of Ip6k1 alleles from IP6K1-KO mice generated by the CRISPR/Cas9 system are displayed. The start codon (ATG) underlined in green is disrupted in IP6K1-KO mice. A 73-bp, out-of-frame deletion within Ip6k1 exon 2 in IP6K1-KO mice is indicated by dashes.
(B) Representative PCR genotyping results for Ip6k1 WT, homozygous (KO), and heterozygous (Het) mice.
(C) Immunoblot analysis shows depletion of IP6K1 protein from brain tissues of IP6K1-KO mice. CX, cortex; CB, cerebellum; HIP, hippocampus.
(D) WT and IP6K1-KO hippocampal neurons were cultured and labeled with [3H]myo-inositol, and intracellular inositol phosphates were resolved by HPLC. Quantification indicates ~60% reduction of IP7 in IP6K1-KO hippocampal neurons.
(E and F) Nissl staining shows normal brain structures of 5-week-old, IP6K1-KO mice (E). Representative images of hippocampal regions are shown in (F). Scale bar, 200 μm.
Enhanced Excitatory Synaptic Vesicle Release of IP6K1-KO Hippocampal Neurons
To assess the functional effect of IP6K1 deletion on synaptic transmission ex vivo, we recorded excitatory postsynaptic currents (EPSCs) evoked by electrical stimulation of Schaffer collateral pathways 100 times at various frequencies (10, 20, and 50 Hz) in acute hippocampal brain slices from IP6K1-KO and WT mice. A plot of normalized evoked EPSC (eEPSC) amplitudes against time at each frequency revealed short-term synaptic plasticity in hippocampi from both genotypes. Interestingly, the rate of short-term synaptic facilitation was greater in WT mice than IP6K1-KO mice at stimulation frequencies of 20 and 50 Hz stimulation (p > 0.11 for 10 Hz; p < 0.05 for 20 Hz, p < 0.001 for 50 Hz) (Figures 2A and 2B). Given that the balance between residual Ca2+ and initial release probability determines short-term synaptic plasticity, presynaptic release probability is likely to be increased in IP6K1-KO mice. Consistent with this, paired-pulse ratio (PPR) was significantly decreased upon higher frequency stimulation (F(1,68) = 20.504, p < 0.0001 for genotype; F(2,68) = 3.728, p < 0.05 for frequency) (Figure 2C). Besides the PPR, we calculated the release probability by the amplitude of first eEPSC divided by the size of readily releasable synaptic vesicle pool (RRP). RRP size was estimated by using back extrapolation of cumulative eEPSC charge transfer during sustained stimulus (20 Hz) (Lou et al., 2012, Macia et al., 2006, Thanawala and Regehr, 2013). Consistent with our observations with release probability measured by PPR, the calculated release probability was significantly increased in IP6K1-KO mice (Figure 2D, p < 0.05). The current findings thus suggest that IP6K1 may be one of the physiological regulators involved in clamping synaptic exocytosis and show that the impact of IP6K1 deletion on short-term synaptic plasticity is activity dependent.
Figure 2.
Evoked Excitatory Presynaptic Vesicle Release is Enhanced in Hippocampal Neurons from IP6K1-KO Mice
(A) Normalized eEPSC amplitudes in hippocampal neurons from IP6K1-KO mice decreased markedly faster than those from WT mice following 100 pulses of 20 Hz or 50 Hz stimulation, but not 10 Hz stimulation (10 Hz stimulation, n = 7–11, minimum p > 0.11; 20 Hz stimulation, n = 7–12, ∗p < 0.05 for stimuli 3-4; 50 Hz stimulation, n = 4–9, ∗p < 0.05 for stimuli 15-31, ∗∗p < 0.01 for stimuli 8-14, ∗∗∗p < 0.001 for stimuli 2-4). Scale bars for representative traces: 100 pA and 50 ms.
(B) Maximum facilitation index following 20 Hz stimulation was significantly decreased in IP6K1-KO mice (∗p < 0.05).
(C) PPR was significantly decreased in an activity-dependent manner (two-way ANOVA analysis, F(1,49) = 20.504, ∗∗∗p < 0.001 for genotype; F(2,49) = 3.728, ∗p < 0.05 for frequency).
(D) Calculated release probability was enhanced in IP6K-KO mice (∗p < 0.05).
(E) Frequencies and amplitudes of mEPSCs were not altered in IP6K1-KO mice (n = 5 for each group; p > 0.53 for frequency; p > 0.30 for amplitude). Scale bars for representative traces: 50 pA and 1 s.
(F) There was no alteration in A/N ratio (n = 4–7, p > 0.69). Scale bars for representative traces: 50 pA and 50 ms.
We further found that the frequency and amplitude of miniature EPSCs (mEPSCs) were not different between IP6K1-KO and WT mice (p > 0.53 for frequency; p > 0.30 for amplitude) (Figure 2E), suggesting that spontaneous synaptic transmission is minimally affected by deletion of IP6K1.
To examine whether deficiency of IP6K1 leads to postsynaptic changes, we measured the relative contribution of two major glutamate receptors, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and N-methyl-D-aspartate receptor (NMDAR), on excitatory currents by recording eEPSCs at −60 mV and +40 mV and calculating the ratio of AMPAR- and NMDAR-mediated currents (A/N ratio). There was no difference in the A/N ratio between IP6K1-KO and WT mice (p > 0.69, Figure 2F).
Presynaptic Release Probability Alteration in Hippocampal Inhibitory Synapses in IP6K1-KO Mice
Next, we measured evoked inhibitory postsynaptic currents (eIPSCs) to determine the role of IP6K1 on synaptic vesicle recycling in inhibitory synapses. PPRs of eIPSCs altered compared between genotypes (p < 0.05 for 10 Hz; p > 0.5 for 20 Hz) (Figures S6A and S6B). PPRs in GABAergic synapses were significantly increased in IP6K1-KO mice, suggesting decreased synaptic vesicle releases. These observations indicate that deletion of IP6K1 induces alteration of synaptic transmission in both excitatory and inhibitory synapses, although the level of changes were a lot smaller in inhibitory synapses, suggesting that the action of IP6K1 may differ depending on the availability of its interacting partners at different types of synapses.
Restoration of Presynaptic Release Probability and Short-Term Plasticity by Expression of WT-IP6K1 in IP6K1-KO Mice
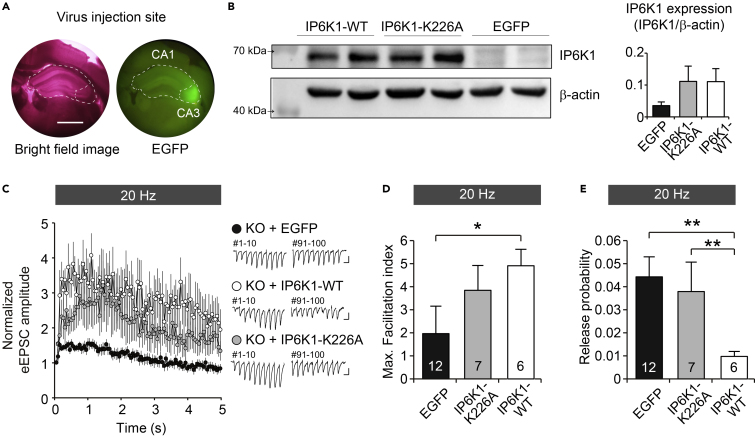
To investigate whether the local expression of IP6K1 in the hippocampus restores short-term synaptic plasticity as shown in WT hippocampus, we virally delivered AAV-IP6K1-WT or AAV-EGFP in the hippocampal CA3 regions of IP6K1-KO mice. After four weeks of injection, we observed ample amount of IP6K1 expression in the whole hippocampus obtained from IP6K1-KO mice (Figures 3A and 3B). Upon sustained electrical stimulus (20 Hz, 100 times), IP6K1-WT-injected KO mice showed restored short-term plasticity and presynaptic release probability in Schaffer collateral pathway (Figures 3C–3E, F(2,22) = 3.547, p < 0.05 for facilitation index, F(2,22) = 6.534, p < 0.01 for release probability).
Figure 3.
Hippocampus-specific Expression of IP6K1 Restored Synaptic Changes Observed in IP6K1-KO Mice
(A) Representative images for virus injection site. The bright field image of hippocampus containing brain slice (left). EGFP-labeled hippocampal CA3 regions of IP6K1-KO mice by AAV-WT-IP6K1 or IP6K1-K/A and AAV-EGFP control virus injection (right). Scale bar, 1 mm.
(B) Immunoblot analysis shows restoration of IP6K1 protein from hippocampal tissues of AAV injected IP6K1-KO mice (n = 2–3).
(C) Normalized eEPSC amplitudes from IP6K1-KO mice after expression of IP6K1 (IP6K1-WT) or catalytically inactive form of IP6K1 (IP6K1-K226A). Facilitation of eEPSC amplitude was significantly enhanced in IP6K1-WT-injected IP6K1-KO mice following 100 pulses of 20 Hz stimulation. IP6K1-K226A-expressing mice showed moderate facilitation of eEPSC (IP6K-WT versus EGFP, n = 6–12, ∗p < 0.05 for stimuli 3, ∗∗p < 0.01 for stimuli 28-100, ∗∗∗p < 0.001 for stimuli 4-27; IP6K1-K226A versus EGFP, n = 7–12, ∗p < 0.05 for stimuli 4-99). Scale bars for representative traces: 100 pA and 50 ms.
(D) Maximum facilitation index following 20 Hz stimulation was significantly increased in IP6K1-WT-expressing KO mice (One-way ANOVA analysis, n = 6–12, F(2,22) = 3.547, ∗p < 0.05).
(E) Calculated release probability was decreased in IP6K1-WT-expressing KO mice (one-way ANOVA analysis n = 6–12, F(2,22) = 6.534, ∗∗p < 0.01), whereas no significant changes were observed between EGFP-injected and IP6K1-K226A-injected KO mice.
In order to examine whether the catalytic activity of IP6K1 is required for IP6K1-dependent synaptic changes, we generated catalytically inactive IP6K1 harboring a single K226A point mutation (Lee et al., 2016). Importantly, enhanced presynaptic release probability seems to remain high even after IP6K1-K226A expression in IP6K1 KO mice (Bonferroni post hoc., EGFP KO versus IP6K1-K226A-injected KO, p > 0.09; IP6K1-WT versus IP6K1-K226A-injected KO, p < 0.01), validating that IP6K1's product, 5-IP7, plays a role in suppressing presynaptic exocytosis. Interestingly, IP6K1-K226A-injected KO mice showed partially restored short-term plasticity (Bonferroni post hoc., EGFP KO versus IP6K1-K226A-injected KO, p > 0.9; IP6K1-WT versus IP6K1-K226A-injected KO, p > 0.2). Our rescue experiments thus revealed that IP6K1 regulates both synaptic vesicle release probability and short term facilitation; however, the catalytic activity of IP6K1 is required for regulation of the release probability but not for short-term facilitation.
Suppressed Synaptic Vesicle Resupply after Vesicle Exhaustion in IP6K1-KO Neurons
To further investigate the impact of IP6K1 deletion on synaptic vesicle trafficking, we challenged presynaptic terminals of the hippocampus with excessive electrical stimulation (20 Hz for 15 s), followed by 1-Hz stimulation. Trains of stimulation at 20 Hz resulted in successful temporary depletion of available vesicles in both WT and IP6K1-KO mice (p > 0.22; Figure 4A). After synaptic vesicle exhaustion, we measured eEPSC amplitude during 1-Hz stimulations for 3 min to allow restoration of synaptic responses. In WT mice, synaptic responses recovered to ∼70% of their initial amplitude in 50 s and fully recovered at the end of 1-Hz trains (p > 0.99; Figures 4A and 4C). In the case of IP6K1-KO hippocampal slices, however, synaptic responses recovered to ∼40% of their initial amplitude in 50 s and ∼60% at the end of 1-Hz trains (p < 0.01; Figures 4A and 4C), indicating that the magnitude of restored responses was significantly reduced in IP6K1-KO mice (p < 0.05; Figure 4D). These observations show that IP6K1 deletion limits synaptic vesicle resupply to the recycling pool of synaptic vesicles following intensive challenge. This was not attributable to differences in initial eEPSC amplitudes, because the averaged initial eEPSC amplitudes were not significantly different between genotypes (p > 0.19; Figure 4B). To quantitatively compare rates of synaptic depression and synaptic recovery, we fitted normalized graphs for the falling phase and rising phase to a single exponential function. Time constants of the falling phase (p > 0.68) and rising phase (p > 0.32) were not different between genotypes (Figure 4E), suggesting that synaptic vesicles cannot be fully restored after synaptic vesicle exhaustion in the context of IP6K1 deletion.
Figure 4.
Synaptic Vesicle Resupply after Exhaustion is Suppressed in Hippocampal Neurons from IP6K1-KO Mice
(A) Normalized eEPSC amplitudes were substantially depressed during 20-Hz stimulation and gradually increased during 1-Hz stimulation (n = 9–16).
(B) Average initial eEPSC amplitudes were comparable between genotypes (p > 0.19).
(C) The magnitude of eEPSC depression was comparable in IP6K1-KO (n = 16) and WT (n = 9) mice (p > 0.22).
(D) The magnitude of eEPSC restoration was significantly reduced in IP6K1-KO mice (∗p < 0.05).
(E) Time constants (τ) of synaptic vesicle exhaustion and resupply were not different between IP6K1-KO and WT mice (p > 0.68 for τ during exhaustion; p > 0.32 for τ during resupply). Representative fits to single-exponential functions for WT mice (orange) and IP6K1-KO mice (blue) are shown for synaptic vesicle exhaustion (WT, τ =23.51; KO, τ =21.17) and synaptic vesicle resupply (WT, τ =44.52; KO, τ =60.97). Scale bars for representative traces: 100 pA and 50 ms.
Impaired Vesicle Reuse During Exocytosis in IP6K1-KO Neurons
Previous studies using in vitro assays have reported that IPs such as IP6 bind and functionally interrupt clathrin assembly proteins (APs), such as AP-2 and AP-3, which are instrumental in vesicle endocytosis (Norris et al., 1995, Voglmaier et al., 1992, Ye et al., 1995). IP6-regulated downstream signaling events were also found to control synaptic vesicle proteins required for endocytosis (Hilton et al., 2001). In pancreatic beta cells, IP6 appears to promote dynamin-mediated endocytosis in association with insulin exocytosis (Hoy et al., 2002). Does IP6K1 deficiency have any impact on synaptic vesicle endocytosis per se at central synapses? To address this question, we employed an additional pharmacological manipulation to isolate exocytosis and endocytosis during recordings (Figure 5A). Folimycin is a vacuolar-ATPase inhibitor that decreases synaptic output by interrupting the neurotransmitter refilling step (Sankaranarayanan and Ryan, 2001). To limit synaptic vesicle reuse, we recorded evoked excitatory responses in the absence or presence of folimycin while stimulating at 20 Hz. In WT animals, treatment of hippocampal slices with folimycin (80 nM for 10 min) caused use-dependent synaptic depression of eEPSCs compared with that in pre-folimycin treatment controls (p < 0.01; Figure 5B), as reported previously (Ertunc et al., 2007). The difference in eEPSC amplitudes before and after folimycin treatment reflects the amount of synaptic vesicle endocytosis that occurred during stimulation, supporting previous observations that recycled vesicles are rapidly recruited for synaptic transmission (Ertunc et al., 2007). However, folimycin treatment in IP6K1-KO mice did not reveal any involvement of endocytic components during 20-Hz stimulation, suggesting that endocytosis during exocytosis might be impaired in these mice (p < 0.05; Figure 5B). There were no alterations in initial eEPSC amplitudes between before and after folimycin treatment (p > 0.08; Figure 5C).
Figure 5.
Synaptic Vesicle Endocytosis and Reuse are Impaired in Hippocampal Neurons from IP6K1-KO Mice
(A and B) (A) Experimental scheme for the vesicle-reuse test. (B) After folimycin treatment (80 nM, 10 min), the rate of eEPSC depression from hippocampal neurons during 20 Hz stimulation in WT mice was significantly faster than that before treatment (n = 4, ∗p < 0.05 for stimuli 15-41 and 50-78). There was no difference in eEPSC depression rate between before and after folimycin treatment in IP6K1-KO mice (n = 8, minimum p > 0.08). Scale bars for representative traces: 100 pA and 50 ms.
(C) Initial eEPSC amplitudes were not different in WT or IP6K1-KO groups (p > 0.63 for WT, p > 0.13 for IP6K1-KO).
(D) Dynasore treatment (80 μM, 10 min) expedite the synaptic depression during 20-Hz stimulation (n = 5–9, ∗p < 0.05 for stimuli 6-20). Dynasore has minimal effect in IP6K1-KO synapses (p > 0.1).
(E) Initial amplitudes of eEPSCs were comparable between WT and IP6K1-KO groups (p > 0.15 for WT, p > 0.84 for IP6K1-KO).
To directly examine the impact of IP6K1 in endocytosis, we employed a small molecule dynamin inhibitor, called dynasore (Macia et al., 2006, Newton et al., 2006). Incubation with 80 μM dynasore induced notable use-dependent short-term depression in WT (p < 0.05; Figure 5D), whereas no difference in IP6K1-KO synapses (p > 0.1; Figure 5D), suggesting that the acute blockade of dynamin function has limited impact in synaptic transmission during high challenges in the absence of IP6K1 as if dynamin has not been working in IP6K1-KO synapses. Taken together, our data suggest that IP6K1 and 5-IP7 metabolism are likely to be involved in regulation of synaptic vesicle endocytosis and coupling between exo- and endocytosis.
Discussion
Tight coupling between exocytosis and endocytosis is critical for coordinating synaptic transmission at central synapses. It has been decades since inositol poly- and pyrophosphates were suggested as key metabolites in the regulation of synaptic vesicle control (Wenk and De Camilli, 2004). Among these IPs, 5-IP7 was recently proposed as the most potent in suppressing synaptic vesicle exocytosis, but these conclusions have been drawn from experiments using cell lines or primary cultures. To define this important issue at a physiological level, we took advantage of mice genetically lacking Ip6k1 and employed electrophysiological approaches to probe the function of IP6K1 in synaptic vesicle recycling in acute hippocampal slices. Our principle findings can be summarized as follows: (1) Measurements of evoked EPSCs revealed that deletion of IP6K1 increased presynaptic release probability, suggesting enhanced excitatory synaptic vesicle fusion. (2) Short-term facilitation was decreased in IP6K1-KO neurons. (3) WT IP6K1, but not catalytically inactive mutant IP6K1, successfully restored a phenotype of presynaptic release probability in IP6K1-KO neurons. (4) Both WT and catalytically inactive IP6K1 recovered short-term facilitation of IP6K1-KO neurons. (5) The IP6K1-KO hippocampus failed to respond to folimycin and dynasore, indicating that endocytosis during exocytosis is severely impaired in IP6K1-KO mice. Collectively, these findings reveal physiological roles of 5-IP7 metabolism in controlling synaptic vesicle exocytosis and endocytosis, as well as their coupling.
A possible mechanistic explanation for the selective enhancement of synaptic vesicle exocytosis in excitatory synaptic function in IP6K1-KO mice is interaction between the IP6K1 product, 5-IP7, and Syt1, a Ca2+ sensor enriched in excitatory synapses (Fernandez-Chacon et al., 2001, Geppert et al., 1994, Rizo and Rosenmund, 2008). Syt1 function can be potently suppressed by its high-affinity ligand, 5-IP7, which interacts with the polybasic C2B domain of Syt1 to reduce synaptic vesicle fusion (Lee et al., 2016). Cultured hippocampal neurons harboring a mutant form of Syt1 with a point mutation in the Ca2+-sensing domain exhibit impaired neurotransmitter release and abnormally facilitated synaptic responses to successive stimulation (Fernandez-Chacon et al., 2001). Taken together, these observations suggest that depletion of the Syt1 inhibitor 5-IP7 by IP6K1-KO contributes to the facilitation of synaptic vesicle fusion.
Interestingly, we found IP6K1 regulates release probability and short-term facilitation in two distinct mechanisms. When we fully restored IP6K1 in KO hippocampus, both release probability and short-term facilitation were recovered to the levels of WT. However, expression of catalytically inactive form of IP6K1 failed to rescue presynaptic release probability in the IP6K1-KO neurons, clearly demonstrating that IP6K1's product, 5-IP7, is the key factor in the control of presynaptic vesicle exocytosis (Figure 3). Introduction of catalytically inactive mutant IP6K1 could restore short-term facilitation to the levels comparable to WT (Figure 3), suggesting a non-catalytic action of IP6K1 enzyme in the regulation of presynaptic events.
IP6K1 is known to directly interact with GRAB, a GDP-GTP exchange factor for a subset of Rab GTPases, including Rab3 and Rab8, and this interaction does not require catalytic activity (Guo et al., 2013, Luo et al., 2001). GSK3 is also known as activated by non-catalytic interactions between IP6K1 and GSK3 (Chakraborty et al., 2010, Chakraborty et al., 2014). Therefore, IP6K1 appears to interact with synaptic proteins regardless of its catalytic activity, which may be sufficient to restore short-term facilitation but not release probability. Several studies suggest the role of GSK3 and Rab GTPase in short-term synaptic plasticity. GSK3 is known to directly phosphorylate and modulate dynamin (Clayton et al., 2010), a GTPase required for synaptic vesicle fission events. Inhibition of dynamin phosphorylation enhances short-term synaptic depression upon high-frequency stimulation (Fà et al., 2014). Moreover, Rab3A is involved in short-term synaptic facilitation. Mutation or deletion of Rab3A protein was shown to enhance synaptic facilitation upon repetitive stimulation (Doussau et al., 1998, Geppert et al., 1997, Schluter et al., 2006). These studies suggest that non-catalytic interaction of IP6K1 with GSK3 or GRAB may mediate short-term synaptic facilitation in the hippocampus.
Since the first report by Saiardi et al. (Saiardi et al., 2002) that budding yeast lacking inositol pyrophosphates by the deletion of KCS1, an yeast ortholog of mammalian IP6K, exhibit defective endocytic trafficking and a fragmented vacuolar morphology, it has been speculated that 5-IP7 and IP6K1 play a role in controlling endocytosis. However, the physiological actions of 5-IP7 metabolism for the control of endocytic events in mammals have remained unanswered. Here, we demonstrate that endocytosis of synaptic vesicles at central synapses is markedly impaired in the hippocampus upon IP6K1 deletion (Figures 4 and 5), establishing IP6K and its product, 5-IP7, as key physiological regulators of endocytic membrane trafficking events from yeast to mammals. With diminished synaptic vesicle reuse after vesicle exhaustion, IP6K1-KO hippocampal neurons exhibited a striking absence of sensitivity to the vacuolar ATPase inhibitor, folimycin, and dynamin inhibitor, dynasore, indicating that 5-IP7 metabolism is essential for proper presynaptic vesicle endocytosis.
How might IP6K1 deletion influence synaptic endocytosis during exocytosis? Our current observations bear a remarkable resemblance to studies using dynamin 1-deficient animals (Ferguson et al., 2007, Hayashi et al., 2008). Cortical synapses obtained from dynamin 1-deleted mice exhibit impaired, activity-dependent synaptic vesicle recycling largely owing to incomplete endocytosis during activity (Ferguson et al., 2007, Hayashi et al., 2008). In support of this idea, in pancreatic beta cells, the 5-IP7 precursor, IP6, was found to control protein kinase C intracellular signaling cascades to drive the formation of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), a key lipid metabolite that triggers formation of clathrin-coated pits (Hoy et al., 2002). In addition to direct binding to target proteins, 5-IP7 may also modulate protein functions by conferring a posttranslational modification known as pyrophosphorylation, which involves the transfer of a high-energy β-phosphate from 5-IP7 to specific proteins (e.g., AP3 beta subunit, dynein intermediate chain) (Azevedo et al., 2009, Chanduri et al., 2016, Park et al., 2018). Identification of 5-IP7-binding and pyrophosphorylation targets in the presynaptic terminal awaits further investigation.
Pharmacological blockade of dynamin phosphorylation by GSK3 impairs synaptic vesicle endocytosis in hippocampal synapses (Clayton et al., 2010). Thus, decreased GSK3 signaling in IP6K1-KO mice may partially mimic the blockade of dynamin phosphorylation and could thus account for the observed impairment in endocytosis after excessive challenge (Figure 4). We should also consider competition between the IP6K1 catalytic product, 5-IP7, and PI(4,5)P2. Given that AP2 and AP180 are ligands for 5-IP7 that bind with sub-nanomolar affinities (Norris et al., 1995, Voglmaier et al., 1992, Ye et al., 1995), the interplay among 5-IP7, clathrin adaptor proteins, and plasma membrane phosphoinositides (e.g., PI(4,5)P2) might be another route to endocytic regulation. The direct binding of GRAB to Rab11, a player in the control of activity-dependent bulk endocytosis (Horgan et al., 2013, Kokotos et al., 2019), further implies complex regulation of presynaptic vesicle trafficking by IP6K1-GRAB-Rab signaling cascades.
In conclusion, our findings unveil the physiological significance of the mammalian 5-IP7 biosynthesis metabolism in controlling presynaptic vesicle cycling involving both exocytic and endocytic trafficking. Dysregulated IP metabolism in humans and genetic deletion of IP6K enzymes in mice have been linked to numerous psychiatric and neural diseases (Belmaker et al., 1996, Chakraborty et al., 2014, Nagata et al., 2011). Our next challenge will be to not only elucidate the molecular details of how IP6K1 and 5-IP7 dynamically fine-tune synaptic vesicle exocytosis-endocytosis but also develop therapeutic strategies for controlling the release of neurotransmitters at central synapses.
Limitations of the Study
The expression of catalytically inactive mutant form of IP6K1 in Ip6k1-KO hippocampal neurons showed partial rescue of the Ip6k1-KO phenotypes, clearly suggesting that IP6K1 plays both 5-IP7-dependent and 5-IP7-independent functions at the central synaptic vesicle cycling events. Further works will be needed to validate IP6K1's non-catalytic actions. Additional experiments are also necessary to reveal the exact site and mode of IP6K1 action in the control of synaptic vesicle endocytosis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Jina Park and all the members of the Kim and Chung labs as well as Ms. Seung Min Park for discussion and helpful comments. This work was supported by the National Research Foundation of Korea (NRF-2013M3C7A1056102, NRF-2018R1A5A1024261, NRF-2018R1A2B2005913 to S.K.; NRF-2017R1A2B4006535, NRF-2015M3C7A1031395 to C.C.), TJ Park Science Fellowship of the POSCO TJ Park Foundation (to S.E.P.), and KAIST Advanced Institute for Science-X fellowship (to S.J.P.).
Author Contributions
S.J.P., H.P., S.K., and C.C. conceived and designed the experiments. S.J.P., H.P., M.G.K., S.Z., and S.E.P. performed the experiments. S.J.P., H.P., S.K., and C.C. analyzed the data and co-wrote the paper. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101000.
Contributor Information
Seyun Kim, Email: seyunkim@kaist.ac.kr.
ChiHye Chung, Email: cchung@konkuk.ac.kr.
Supplemental Information
References
- Azevedo C., Burton A., Ruiz-Mateos E., Marsh M., Saiardi A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proc. Natl. Acad. Sci. U S A. 2009;106:21161–21166. doi: 10.1073/pnas.0909176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker R.H., Bersudsky Y., Agam G., Levine J., Kofman O. How does lithium work on manic depression? Clinical and psychological correlates of the inositol theory. Annu. Rev. Med. 1996;47:47–56. doi: 10.1146/annurev.med.47.1.47. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Koldobskiy M.a, Bello N.T., Maxwell M., Potter J.J., Juluri K.R., Maag D., Kim S., Huang A.S., Dailey M.J. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A., Kim S., Snyder S.H. Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 2011;4:re1. doi: 10.1126/scisignal.2001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A., Latapy C., Xu J., Snyder S.H., Beaulieu J.-M. Inositol hexakisphosphate kinase-1 regulates behavioral responses via GSK3 signaling pathways. Mol. Psychiatry. 2014;19:284–293. doi: 10.1038/mp.2013.21. [DOI] [PubMed] [Google Scholar]
- Chanduri M., Rai A., Malla A.B., Wu M., Fiedler D., Mallik R., Bhandari R. Inositol hexakisphosphate kinase 1 (IP6K1) activity is required for cytoplasmic dynein-driven transport. Biochem. J. 2016;473:3031–3047. doi: 10.1042/BCJ20160610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E.L., Sue N., Smillie K.J., O’Leary T., Bache N., Cheung G., Cole A.R., Wyllie D.J., Sutherland C., Robinson P.J. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat. Neurosci. 2010;13:845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussau F., Clabecq A., Henry J., Francois D., Poulain B. Calcium-dependent regulation of Rab3 in short-term plasticity. J. Neurosci. 1998;18:3147–3157. doi: 10.1523/JNEUROSCI.18-09-03147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertunc M., Sara Y., Chung C., Atasoy D., Virmani T., Kavalali E.T. Fast synaptic vesicle reuse slows the rate of synaptic depression in the CA1 region of hippocampus. J. Neurosci. 2007;27:341–354. doi: 10.1523/JNEUROSCI.4051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fà M., Staniszewski A., Saeed F., Francis Y.I., Arancio O. Dynamin 1 is required for memory formation. PLoS One. 2014;9:e91954. doi: 10.1371/journal.pone.0091954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., Raimondi A., Gong L., Ariel P., Paradise S. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R., Konigstorfer A., Gerber S.H., Garcia J., Matos M.F., Stevens C.F., Brose N., Rizo J., Rosenmund C., Sudhof T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;233:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fu C., Xu J., Cheng W., Rojas T., Chin A.C., Snowman A.M., Harraz M.M., Snyder S.H. Neuronal migration is mediated by inositol hexakisphosphate kinase 1 via α-actinin and focal adhesion kinase. Proc. Natl. Acad. Sci. U S A. 2017;114:2036–2041. doi: 10.1073/pnas.1700165114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R.E., Li C., RosahI T.W., Stevens C.F., Sudhof T.C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:19717–19727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Stevens C.F., Sudhof T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Guo Z., Hou X., Goody R.S., Itzen A. Intermediates in the guanine nucleotide exchange reaction of Rab8 protein catalyzed by guanine nucleotide exchange factors Rabin8 and GRAB. J. Biol. Chem. 2013;288:32466–32474. doi: 10.1074/jbc.M113.498329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch A.J., York J.D. SnapShot: inositol phosphates. Cell. 2010;143:1030–1030.e1. doi: 10.1016/j.cell.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Raimondi A., O’Toole E., Paradise S., Collesi C., Cremona O., Ferguson S.M., De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc. Natl. Acad. Sci. U S A. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J.M., Plomann M., Ritter B., Modregger J., Freeman H.N., Falck J.R., Krishna U.M., Tobin A.B. Phosphorylation of a synaptic vesicle-associated protein by an inositol hexakisphosphate-regulated protein kinase. J. Biol. Chem. 2001;276:16341–16347. doi: 10.1074/jbc.M011122200. [DOI] [PubMed] [Google Scholar]
- Horgan C.P., Hanscom S.R., Mccaffrey M.W. GRAB is a binding partner for the Rab11a and Rab11b GTPases. Biochem.Biophys. Res. Commun. 2013;441:214–219. doi: 10.1016/j.bbrc.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Hoy M., Efanov A.M., Bertorello A.M., Zaitsev S.V., Olsen H.L., Bokvist K., Leibiger B., Leibiger I.B., Zwiller J., Berggren P.-O. Inositol hexakisphosphate promotes dynamin I-mediated endocytosis. Proc. Natl. Acad. Sci. U S A. 2002;99:6773–6777. doi: 10.1073/pnas.102157499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F., Schell M.J. Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- Kokotos A.C., Peltier J., Davenport E.C., Trost M., Cousin M.A. Activity-dependent bulk endocytosis proteome reveals a key presynaptic role for the monomeric GTPase Rab11. Proc. Natl. Acad. Sci. U S A. 2019;116:2386–2388. doi: 10.1073/pnas.1809189115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-S., Lee J.-Y., Kyung J.W., Yang Y., Park S.J., Lee S., Pavlovic I., Kong B., Jho Y.S., Jessen H.J. Inositol pyrophosphates inhibit synaptotagmin-dependent exocytosis. Proc. Natl. Acad. Sci. U S A. 2016;113:8314–8319. doi: 10.1073/pnas.1521600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lang E.J., Morita M., Fukuda M., Niinobe M., Mikoshiba K. The inositol high-polyphosphate series blocks synaptic transmission by preventing vesicular fusion: a squid giant synapse study. Proc. Natl. Acad. Sci. U S A. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X., Fan F., Messa M., Raimondi A., Wu Y., Looger L.L., Ferguson S.M. Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses. Proc. Natl. Acad. Sci. U S A. 2012;109:515–523. doi: 10.1073/pnas.1121626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H.R., Saiardi A., Nagata E., Ye K., Yu H., Jung T.S., Luo X., Jain S., Sawa A., Snyder S.H. GRAB: a physiologic guanine nucleotide exchange factor for Rab3A , which interacts with inositol hexakisphosphate kinase. Neuron. 2001;31:439–451. doi: 10.1016/s0896-6273(01)00384-1. [DOI] [PubMed] [Google Scholar]
- Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Nagata E., Saiardi A., Tsukamoto H., Okada Y., Itoh Y., Satoh T., Itoh J., Margolis R.L., Takizawa S., Sawa A. Inositol hexakisphosphate kinases induce cell death in Huntington disease. J. Biol. Chem. 2011;286:26680–26686. doi: 10.1074/jbc.M111.220749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A.J., Kirchhausen T., Murthy V.N. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc. Natl. Acad. Sci. U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris F.A., Ungewickell E., Majerus P.W. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP- 3/AP180) and inhibits clathrin cage assembly in vitro. J. Biol. Chem. 1995;270:214–217. doi: 10.1074/jbc.270.1.214. [DOI] [PubMed] [Google Scholar]
- Park S.J., Lee S., Park S.E., Kim S. Inositol pyrophosphates as multifaceted metabolites in the regulation of mammalian signaling networks. Anim. Cells Syst. 2018;22:1–6. [Google Scholar]
- Rizo J., Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiardi A., Erdjument-Bromage H., Snowman A.M., Tempst P., Snyder S.H. Synthesis of diphosphoinositolpentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr. Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- Saiardi A., Sciambi C., McCaffery J.M., Wendland B., Snyder S.H. Inositol pyrophosphates regulate endocytic trafficking. Proc. Natl. Acad. Sci. U S A. 2002;99:14206–14211. doi: 10.1073/pnas.212527899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S., Ryan T.A. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schluter O.M., Basu J., Sudhof T.C., Rosenmund C. Rab3 superprimes synaptic vesicles for release: Implications for short-term synaptic plasticity. J. Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F.E., Ryan T.A. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr.Opin.Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Seeds A.M., Frederick J.P., Tsui M.M.K., York J.D. Roles for inositol polyphosphate kinases in the regulation of nuclear processes and developmental biology. Adv. Enzyme Regul. 2007;47:10–25. doi: 10.1016/j.advenzreg.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S.B. Inositol pyrophosphates: why so many phosphates? Adv. Biol. Regul. 2015;57:203–216. doi: 10.1016/j.jbior.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Thanawala M.S., Regehr W.G. Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool. J. Neurosci. 2013;33:4625–4633. doi: 10.1523/JNEUROSCI.4031-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier S.M., Keen J.H., Murphy J.E., Ferris C.D., Prestwich G.D., Snyder S.H., Theibert A.B. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem.Biophys. Res. Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- Wenk M.R., De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: Insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U S A. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S.C., Livermore T.M., Saiardi A. Inositol pyrophosphates: between signalling and metabolism. Biochem. J. 2013;452:369–379. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]
- Yang S.-N., Shi Y., Yang G., Li Y., Yu L., Shin O.-H., Bacaj T., Südhof T.C., Yu J., Berggren P.-O. Inositol hexakisphosphate suppresses excitatory neurotransmission via synaptotagmin-1 C2B domain in the hippocampal neuron. Proc. Natl. Acad. Sci. U S A. 2012;109:12183–12188. doi: 10.1073/pnas.1115070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Ali N., Bembenek M.E., Shears S.B., Lafer E.M. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J. Biol. Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.