Abstract
MicroRNAs (miRNAs/miRs) have important roles in tumor progression in various human cancers. Ultrasound-targeted microbubble destruction (UTMD)-mediated gene transfection has been considered a useful tool for improving cancer treatment. The present study aimed to investigate the role of miR-767 in non-small cell lung cancer (NSCLC) and further analyze the effects of UTMD-mediated miR-767 inhibition on tumor progression. The expression of miR-767 was measured by reverse transcription-quantitative PCR. UTMD-mediated miR-767 inhibition was achieved by the co-transfection of microbubbles and miR-767 inhibitor in NSCLC cells. Cell proliferation was assessed by a CCK-8 assay and cell migration and invasion were examined by a Transwell assay. The expression of miR-767 was increased in NSCLC serum, tissues and cells compared with controls. The reduction of miR-767 in NSCLC cells led to the inhibition of cell proliferation, migration and invasion. UTMD increased the transfection efficiency of the miR-767 inhibitor in NSCLC cells, and UTMD-mediated miR-767 inhibition resulted in a more significant suppressive effect on tumor cell proliferation, migration and invasion. Taken together, the results indicated that miR-767 expression is upregulated in both NSCLC clinical samples and cells. The downregulation of miR-767 can inhibit tumor cell proliferation, migration and invasion, and these effects are further promoted by UTMD-mediated miR-767 inhibition, which indicated the potential of a UTMD-mediated miR-767 inhibition as a novel therapeutic strategy for NSCLC treatment.
Keywords: non-small cell lung cancer, microRNA-767, ultrasound-targeted microbubble destruction, proliferation, migration, invasion
Introduction
Lung cancer is the most frequent malignancy worldwide and one of the leading causes of global mortality arising from cancer (1). It can be divided into two subtypes: Small cell lung cancer and non-small cell lung cancer (NSCLC), which are defined based on their histological characteristics (2). NSCLC is considered to be the most common type of lung cancer, accounting for approximately 85% of all lung tumors (3). According to statistics, most patients with NSCLC are diagnosed with advanced tumors due to limited strategies available for early diagnosis (4). Although advancements have been made in terms of therapeutic methods such as surgery, chemotherapy and radiotherapy, the 5-year overall survival of NSCLC is only 11% (5). Thus, there is a need for more efficient strategies to improve the diagnosis, prognosis and therapy for patients with NSCLC.
MicroRNAs (miRNAs/miRs) are found to be deregulated in various human cancers (6). These small noncoding RNAs can regulate gene expression at the post-transcriptional level and are involved in various cell processes such as proliferation, migration, invasion, differentiation and apoptosis (7,8). Emerging studies focused on the clinical significance of miRNAs for their diagnostic and prognostic value (9). In addition, the aberrant expression of miRNAs was reported to play a regulatory role in tumor progression in different types of human cancer (10). Several functional miRNAs with critical roles have also been identified in NSCLC. Li et al (3) found that upregulated expression of miR-421 could predict poor prognosis of NSCLC and contribute to tumor cell proliferation, migration and invasion. Jiang et al (11) showed evidence of miR-940 acting as a tumor suppressor by inhibiting NSCLC cell invasion and epithelial-mesenchymal transition via the transforming growth factor-β signaling pathway. The aforementioned studies indicated the considerable potential of miRNAs as biomarkers and therapeutic targets in NSCLC.
Considering the therapeutic potential of miRNAs for the treatment of human cancers, it is crucial to deliver specific miRNAs to targeted areas using a noninvasive approach with relatively high safety and effectiveness. Ultrasound-targeted microbubble destruction (UTMD) is considered a novel strategy for gene delivery (12). During UTMD, the gene is integrated into a microbubble and is then released when the microbubble reaches the targeted area and collapses (13). Microbubble destruction resulting from ultrasound induces an increase in capillary permeability and induces irreversible holes in target cell membranes, contributing to gene transfer into the nucleus and enhanced expression and transfection of the target gene (14). Additionally, gene transfer by UTMD can avoid degradation by lytic enzymes (15). The application of UTMD has been highlighted in cancer treatment, which greatly contributes to targeted cancer therapy (16).
The abnormal expression of miR-767 is closely related to DNA hypomethylation in NSCLC. miR-767 was identified as an upstream regulator of tet methylcytosine dioxygenase (TET) 1 and TET3, which are established tumor suppressors in various tumors, including NSCLC (17,18). The biological function of miR-767 has been identified in human melanoma (19) and glioma (20). However, the precise role of miR-767 in NSCLC remains to be elucidated. The aim of the present study was to investigate the functional role of miR-767 in NSCLC progression. Furthermore, the present study assessed the transfection efficiency of UTMD-mediated transfection of miR-767 into NSCLC cells and the feasibility of UTMD-mediated miR-767 therapy.
Materials and methods
Clinical sample collection
Samples from 108 patients who were diagnosed with NSCLC in Zibo City Linzi District People's Hospital (Shandong, China) between May 2014 and April 2016 were used in the study. The patients included 62 males and 46 females with a mean age of 63.3±13.9 years (range 25-80 years). None of the patients had received any anti-tumor therapy prior to radical resection surgery. Prior to surgery, serum samples were obtained from blood collected from the patients and stored at -80˚C for further use. During surgery, 108 paired tumor and adjacent normal tissues were isolated and stored in liquid nitrogen. In addition, 50 age (mean 62.8±14.2 years) and gender (male:female ratio, 29:21)-matched healthy volunteers were enrolled in the study to provide healthy serum control samples. All participants signed informed consent before sampling. The experimental procedures were approved by the Ethics Committee of Zibo City Linzi District People's Hospital (approval no. 20140922).
Cell culture and transfection
Human NSCLC cell lines A549, H1299 and PC9 and bronchial epithelial cell line 16HBE were purchased from the Shanghai Cell Bank of China. Cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator with 5% CO2 at 37˚C. miR-767 inhibitor (5'-CAUGCUCAGACAACCAUGGUGCA-3') and miRNA negative control (miR-NC; 5'-CAGUACUUUUGUGUAGUACAA-3') were synthesized by Shanghai GenePharma Co., Ltd. miR-767 inhibitor (100 nM) or miR-NC (100 nM) was transfected into A549 and H1299 cells using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h following the manufacturer's protocols. Cells transfected with the transfection reagent alone served as the mock group.
Microbubble preparation and miRNA transfection
Microbubbles were obtained using a previously reported method (21), which was performed by sonication of 0.4 mg/ml 1,2-distearoyl-3-trimethylammoni-umpropane (Avanti Polar Lipids, Inc.) with perfluoropropane gas, 1 mg/ml polyethyleneglycol-2000 stearate (Avanti Polar Lipids, Inc.) and 2 mg/ml distearoylphosphatidylcholine (Avanti Polar Lipids, Alabaster, Inc). miR-767 inhibitor or miR-NC was incubated with the microbubbles for 30 min at 37˚C. The mixture was then added into H1299 and A549 cells for transfection using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA from clinical samples and cells were extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocol. RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara Bio, Inc.) following the manufacturer's instructions. qPCR was performed using a SYBR-Green PCR Master Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) and a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction conditions were as follows: 95˚C for 10 min, followed by 40 cycles of 95˚C for 20 sec, 60˚C for 10 sec, 72˚C for 15 sec. The primer pairs used for the qPCR were: miR-767 forward, 5'-GCCGAGTGCACCATGGTTGT-3' and reverse, 5'-CTCAACTGGTGTCGTGGA-3' and U6 forward, 5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'. miR-767 expression was quantified using the 2-ΔΔCq method and normalized to the internal reference gene U6(22).
Cell proliferation assay
NSCLC cell proliferation was examined using a Cell Counting kit (CCK)-8 assay (Beyotime Institute of Biotechnology). Tumor cells were seeded at a density of 4x105 cells/well in 96-well plates and cultured at 37˚C for 72 h. CCK-8 solution was added into the cells every 24 h with a further 4 h incubation. Cell proliferation was evaluated by reading the absorbance at a wavelength of 450 nm using a microplate reader (Molecular Devices).
Cell migration and invasion assay
NSCLC cell migration and invasion were measured using Transwell chambers with a pore size of 8 µm (Corning Inc.). Chambers were pre-coated with Matrigel (Corning, Inc.) for the invasion assay. Following 48 h of cell transfection, cells (seeded at a density of 5x105 cells/well) in serum-free RPMI-1640 medium were seeded into the upper chamber. The lower chambers contained culture medium supplemented with 10% FBS. After 24 h of incubation at 37˚C, cells in the lower chambers were stained with crystal violet at room temperature for 20 min and observed under an LX71 inverted light microscope (Olympus Corporation). Cells were counted using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Statistical analysis
Data are expressed as the mean ± SD and analyzed using SPSS 18.0 (SPSS Inc.) and GraphPad Prism 5.0 (GraphPad Software, Inc.). Differences between groups were calculated using one-way ANOVA followed by Tukey's post hoc test, non-parametric Wilcoxon signed-rank test or Mann-Whitney U test when appropriate. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-767 in NSCLC
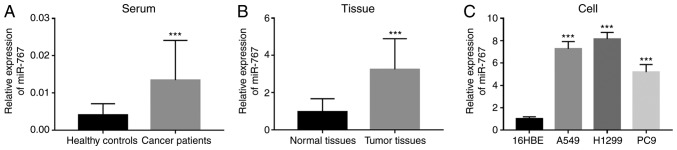
miR-767 expression was measured in clinical samples, including serum and tissue specimens. Significantly increased expression of miR-767 was observed in both serum and tissues of NSCLC patients compared with corresponding normal controls (Fig. 1A and B). In addition, upregulated expression of miR-767 was also found in four NSCLC cell lines (A549, H1299 and PC9) compared with 16HBE cells (Fig. 1C).
Figure 1.
Expression of miR-767 in NSCLC. (A) miR-767 expression is increased in the serum of NSCLC patients (n=108) compared with healthy individuals (n=50). (B) Upregulated expression of miR-767 was observed in NSCLC tissues (n=108) compared with adjacent normal tissues (n=108). (C) Expression of miR-767 was increased in the four tumor cell lines compared with normal cells. All experiments were performed at least three times. ***P<0.001. NSCLC, non-small cell lung cancer; miR-767, microRNA-767.
Knockdown of miR-767 inhibits NSCLC cell proliferation, migration and invasion
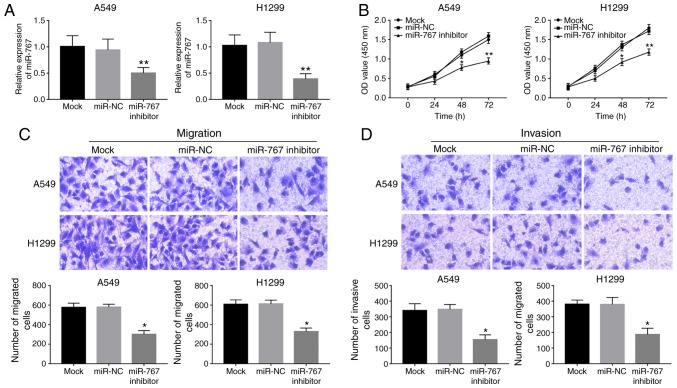
To investigate the biological function of miR-767 in NSCLC progression, in vitro regulation of miR-767 was attained by cell transfection in A549 and H1299 cells. As shown in Fig. 2A, expression of miR-767 was significantly reduced by miR-767 inhibitor transfection in both cell lines. Knockdown of miR-767 in A549 and H1299 cells led to a significant decrease in cell proliferation compared with the mock group (Fig. 2B). Furthermore, cell migration and invasion were suppressed by the inhibition of miR-767 in A549 and H1299 cells compared with mock and miR-NC groups (Fig. 2C and D).
Figure 2.
Effects of miR-767 on cellular processes in the NSCLC cell lines A549 and H1299. (A) Expression of miR-767 decreased following miR-767 inhibitor transfection. (B) Cell proliferation was suppressed by the knockdown of miR-767. Downregulation of miR-767 expression in tumor cells led to inhibited cell (C) migration and (D) invasion (magnification, x200). All experiments were performed at least three times. *P<0.05 and **P<0.01. NSCLC, non-small cell lung cancer; miR-767, microRNA-767; OD, optical density; NC, negative control.
UTMD improves the transfection efficiency of miR-767
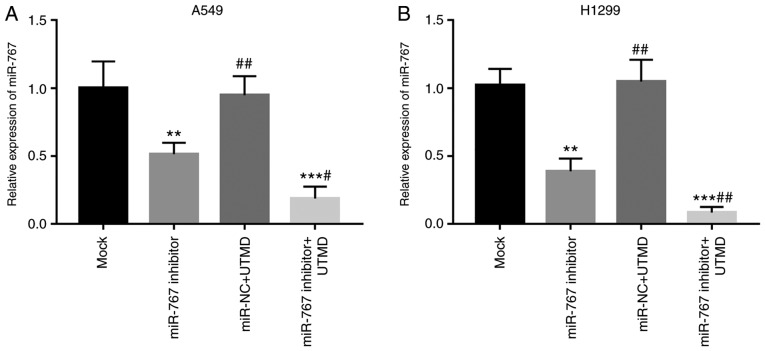
Given the enhancement of gene transfer by UTMD, the present study focused on the effects of UTMD on miR-767 transfection efficiency. Transfection of miR-767 inhibitor with UTMD significantly decreased miR-767 expression compared with mock and miR-767 inhibitor groups in A549 and H1299 cells (Fig. 3).
Figure 3.
UTMD facilitates transfection of miR-767 inhibitor in (A) A549 and (B) H1299 cells. All experiments were performed at least three times. **P<0.01 and ***P<0.001 vs. mock; #P<0.05 and ##P<0.01 vs. miR-767 inhibitor. UTMD, ultrasound-targeted microbubble destruction; miR-767, microRNA-767; NC, negative control.
UTMD-mediated miR-767 inhibition suppresses the proliferation of NSCLC cells
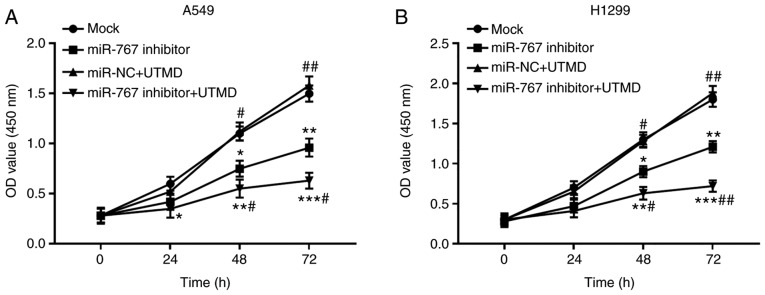
Since the transfection efficiency of miR-767 was enhanced by the application of UTMD, tumor progression was hypothesized to be influenced by UTMD mediation. As shown in Fig. 4, proliferation of A549 and H1299 cells was significantly inhibited by UTMD-mediated miR-767 knockdown compared with the mock group. As expected, a further suppression in cell proliferation was observed in cells transfected with miR-767 inhibitor + UTMD compared with cells transfected with miR-767 inhibitor alone.
Figure 4.
UTMD-mediated miR-767 inhibition further suppressed proliferation of (A) A549 and (B) H1299 cells compared with miR-767 inhibitor transfection alone. All experiments were performed at least three times. *P<0.05, **P<0.01 and ***P<0.001 vs. mock; #P<0.05 and ##P<0.01 vs. miR-767 inhibitor. UTMD, ultrasound-targeted microbubble destruction; miR-767, microRNA-767; NC, negative control; OD, optical density.
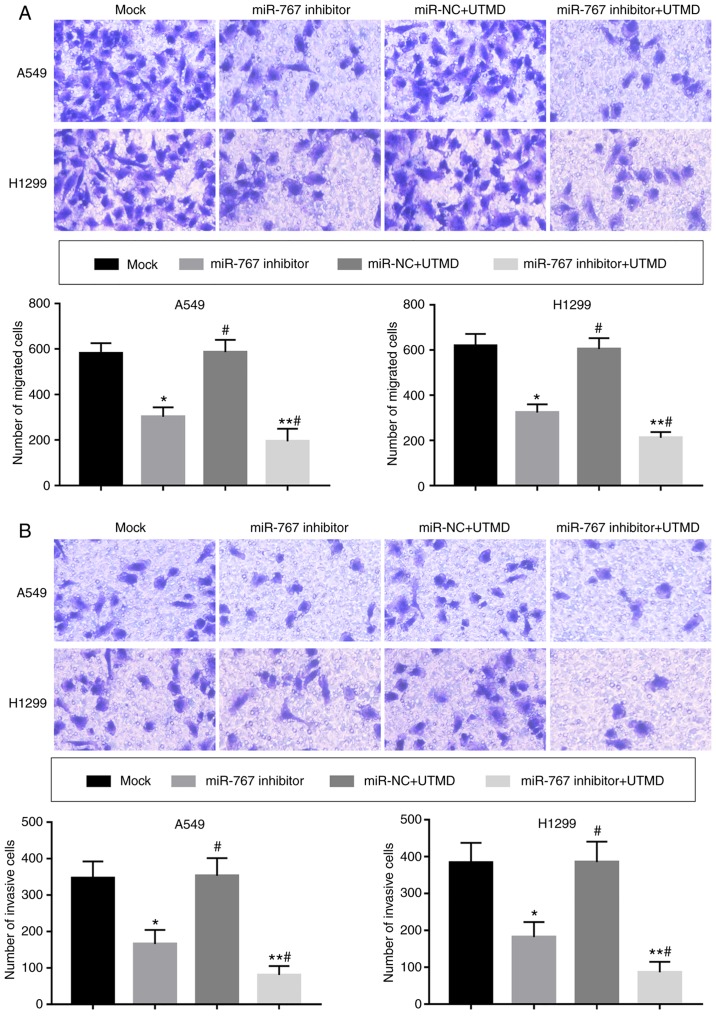
UTMD-mediated miR-767 inhibition inhibits the migration and invasion of NSCLC cells
A significant decrease in cell migration and invasion was observed following in vitro transfection with UTMD-mediated miR-767 inhibitor compared with mock and miR-767 inhibitor groups (Fig. 5).
Figure 5.
UTMD-mediated miR-767 inhibition further inhibited the (A) migration and (B) invasion of A549 and H1299 cells compared with miR-767 inhibitor alone (magnification, x200). All experiments were performed at least three times. *P<0.05 and **P<0.01 vs. mock; #P<0.05 vs. miR-767 inhibitor. UTMD, ultrasound-targeted microbubble destruction; miR-767, microRNA-767; NC, negative control.
Discussion
An increasing number of studies have reported the important role of miRNAs in the treatment of various human cancers (23-25). The present study aimed to investigate the expressional patterns of miR-767 in NSCLC clinical samples and cells and to analyze the functional role of miR-767 in tumor progression. Furthermore, the application of UTMD in miR-767 inhibitor transfection and its effects on the regulation of miR-767 in NSCLC tumor progression were also investigated. Significantly increased expression of miR-767 was found in the serum, tissues and cell lines of NSCLC and knockdown of miR-767 in tumor cells led to inhibited cell proliferation, migration and invasion. The efficiency of the in vitro transfection of miR-767 inhibitor significantly increased by the use of UTMD, and UTMD-mediated miR-767 inhibition resulted in further suppression in NSCLC cell proliferation, migration and invasion.
Numerous miRNAs with aberrant expression have been identified in human cancers (26). The clinical significance of abnormal miRNAs has attracted increasing attention in the diagnosis and prognosis of various cancers (27). Previous findings have shown several miRNAs with ectopic expressional patterns in NSCLC, with demonstrated diagnostic and prognostic value (28). Increased expression of miR-223 in NSCLC sputum samples was identified as a non-invasive diagnostic biomarker for NSCLC detection (29). The ectopic expression of miR-9, miR-16, miR-205 and miR-486 in serum specimens collected from patients with NSCLC served as useful diagnostic biomarkers for NSCLC (30). Upregulated expression of miR-411 was shown to have relatively high diagnostic and prognostic value for patients with NSCLC (31). In addition to the clinical value of miRNAs, their functional roles in tumor progression have also been investigated, providing further evidence for the therapeutic potential of these miRNAs (32). In the pathogenesis of NSCLC, several miRNAs, such as miR-612(33), miR-421(3) and miR-4286(34), were reported to be involved in tumor cell proliferation, migration and invasion and thus may serve as potential therapeutic targets.
Aberrant expression of miR-767 was found to be linked to the hypomethylation of NSCLC (17). In human melanoma, miR-767 was identified as an oncogenic miRNA that promoted tumor cell proliferation (19). In multiple myeloma, miR-767 served as a mediator in the suppressive effects of circ_0000190 on tumor progression (35). In glioma, tumor cell proliferation and migration were inhibited by the overexpression of miR-767, implying the potential of miR-767 as a novel therapeutic target (25). Results from the current study showed increased expression of miR-767 in serum and tissue samples collected from patients with NSCLC. Similarly, the expression of miR-767 was also elevated in NSCLC cell lines compared with normal cells. Thus, miR-767 may have the potential to serve as a biomarker for the screening of NSCLC. Further experiments showed that knockdown of miR-767 induced by miR-767 inhibitor transfection suppressed NSCLC cell proliferation, migration and invasion, indicating that miR-767 might serve as an oncogenic miRNA in the tumor progression of NSCLC.
UTMD is considered an effective method of monitoring the status of tumors and used as a novel tool to facilitate drug or gene transfer by improving the permeability of tumor cells (36). Lin et al (37) reported that UTMD promoted the co-delivery of gemcitabine and miR-21, thereby improving the treatment of pancreatic cancer. Ji et al (38) investigated the application of UTMD in miR-133a delivery and found that UTMD-mediated miR-133a inhibited tumor growth and improved the survival rate in a breast cancer mouse model, suggesting the therapeutic potential of UTMD-mediated miR-133a for breast cancer treatment. UTMD successfully enhanced the transfer of miR-205, as evidenced by the significant increase in miR-205 expression in prostate cancer cells, leading to suppressed tumor cell proliferation, migration and invasion and enhanced cell apoptosis (39). However, to the best of our knowledge, the application of UTMD in the treatment of NSCLC has rarely been reported.
In the present study, the transfection efficiency of the miR-767 inhibitor was enhanced by the use of UTMD in NSCLC cells. An increase in NSCLC cell proliferation, migration and invasion induced by miR-767 inhibitor was further facilitated by UTMD mediation. Therefore, UTMD contributed to in vitro transfection of miR-767 inhibitor, which resulted in enhanced effects of miR-767 on tumor progression of NSCLC. Although the present study provided evidence for the role of miR-767 in NSCLC, the related mechanisms behind its role remain unclear. Loriot et al (17) suggested that miR-767 could directly regulate the expression of TET1 and TET3, which act as tumor suppressors in human cancers (18). In human lung cancer cells, TET1 was shown to inhibit tumor progression by regulating cell migration and invasion (40). Thus, miR-767 might exert its regulatory role in NSCLC progression by targeting TET1 or TET3.
In conclusion, the present study found increased expression of miR-767 in serum and tissues from NSCLC patients and cells compared with corresponding normal controls. Knockdown of miR-767 inhibited tumor cell proliferation, migration and invasion. Furthermore, the transfection efficiency of miR-767 inhibitor can be enhanced by UTMD, resulting in a more significant suppression of the biological functions of NSCLC cells. The results suggested that miR-767 may serve as a promising diagnostic biomarker and a potential therapeutic target in NSCLC, and UTMD-mediated miR-767 inhibitor delivery may be a novel and effective therapeutic strategy for the treatment of NSCLC.
Acknowledgements
The authors would like to thank Dr Min Yang, Dr Yan Su andDr Ruizhu Xie (Affiliated Hospital of Weifang Medical University) for their help in clinical sample collection anddata analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL made conceived and designed the current study, acquired clinical samples and prepared the manuscript preparation. YZ and WL analyzed and interpreted the data and revised the manuscript. MX performed cell experiments.
Ethics approval and consent to participate
All the participants signed the informed consent before sampling. The experimental procedures were approved by the Ethics Committee of Zibo City Linzi District People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(pii: 170070) doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li O, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52:103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejima H, Iinuma H, Kanaoka R, Matsutani N, Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol Lett. 2017;13:1256–1263. doi: 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. EUROCARE-4 Working Group: Recent cancer survival in Europe: A 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zhang X, Yang Z, Li Y, Han B, Chen LA. miR-339-5p inhibits metastasis of non-small cell lung cancer by regulating the epithelial-to-mesenchymal transition. Oncol Lett. 2018;15:2508–2514. doi: 10.3892/ol.2017.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Pan X, Hu Z. MiR-502 mediates esophageal cancer cell TE1 proliferation by promoting AKT phosphorylation. Biochem Biophys Res Commun. 2018;501:119–123. doi: 10.1016/j.bbrc.2018.04.188. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Liang Y, Lv H, Meng H, Xiong G, Guan X, Chen X, Bai Y, Wang K. miR-26a and miR-26b inhibit esophageal squamous cancer cell proliferation through suppression of c-MYC pathway. Gene. 2017;625:1–9. doi: 10.1016/j.gene.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Z, Li H, Wang J, Sun C. miR-146a and miR-146b in the diagnosis and prognosis of papillary thyroid carcinoma. Oncol Rep. 2017;38:2735–2740. doi: 10.3892/or.2017.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Guo Q, Gao S, Hua K. miR-454-3p promotes proliferation and induces apoptosis in human cervical cancer cells by targeting TRIM3. Biochem Biophys Res Commun. 2019;516:872–879. doi: 10.1016/j.bbrc.2019.06.126. [DOI] [PubMed] [Google Scholar]
- 11.Jiang K, Zhao T, Shen M, Zhang F, Duan S, Lei Z, Chen Y. MiR-940 inhibits TGF-β-induced epithelial-mesenchymal transition and cell invasion by targeting Snail in non-small cell lung cancer. J Cancer. 2019;10:2735–2744. doi: 10.7150/jca.31800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Y, Han Z, Shao L, Zhao Y. Ultrasound-targeted microbubble destruction of calcium channel subunit alpha 1D siRNA inhibits breast cancer via G protein-coupled receptor 30. Oncol Rep. 2016;36:1886–1892. doi: 10.3892/or.2016.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Hwang JH. Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J Ther Ultrasound. 2013;1(10) doi: 10.1186/2050-5736-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers. J Pharm Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- 15.Tu J, Zhang H, Yu J, Liufu C, Chen Z. Ultrasound-mediated microbubble destruction: A new method in cancer immunotherapy. Onco Targets Ther. 2018;11:5763–5775. doi: 10.2147/OTT.S171019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Li RK. Ultrasound-targeted microbubble destruction in gene therapy: A new tool to cure human diseases. Genes Dis. 2017;4:64–74. doi: 10.1016/j.gendis.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loriot A, Van Tongelen A, Blanco J, Klaessens S, Cannuyer J, van Baren N, Decottignies A, De Smet C. A novel cancer-germline transcript carrying pro-metastatic miR-105 and TET-targeting miR-767 induced by DNA hypomethylation in tumors. Epigenetics. 2014;9:1163–1171. doi: 10.4161/epi.29628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thienpont B, Galle E, Lambrechts D. TET enzymes as oxygen-dependent tumor suppressors: Exciting new avenues for cancer management. Epigenomics. 2016;8:1445–1448. doi: 10.2217/epi-2016-0126. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Guo L. MiR-767 promoted cell proliferation in human melanoma by suppressing CYLD expression. Gene. 2018;641:272–278. doi: 10.1016/j.gene.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Xu S, Xu J, Li Y, Zhang J, Zhang J, Lu X. miR7675p inhibits glioma proliferation and metastasis by targeting SUZ12. Oncol Rep. 2019;42:55–66. doi: 10.3892/or.2019.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Dou Y, Sui Z, Cheng H, Liu X, Wang Q, Gao P, Qu Y, Xu M. Upregulated miRNA-182-5p expression in tumor tissue and peripheral blood samples from patients with non-small cell lung cancer is associated with downregulated Caspase 2 expression. Exp Ther Med. 2020;19:603–610. doi: 10.3892/etm.2019.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Tang X, Shi X, Su L. miR-532-5p promotes breast cancer proliferation and migration by targeting RERG. Exp Ther Med. 2020;19:400–408. doi: 10.3892/etm.2019.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Kong X, Shi Q, Zhao B. MicroRNA-383-5p acts as a potential prognostic biomarker and an inhibitor of tumor cell proliferation, migration, and invasion in breast cancer. Cancer Biomark: Dec. 2019;24 doi: 10.3233/CBM-190704. doi: 10.3233/CBM-190704 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Biswas S. MicroRNAs as therapeutic agents: The future of the battle against cancer. Curr Top Med Chem. 2018;18:2544–2554. doi: 10.2174/1568026619666181120121830. [DOI] [PubMed] [Google Scholar]
- 27.Azarbarzin S, Hosseinpour Feizi MA, Safaralizadeh R, Ravanbakhsh R, Kazemzadeh M, Fateh A, Karimi N, Moaddab Y. The value of miR-299-5p in diagnosis and prognosis of Intestinal-type gastric adenocarcinoma. Biochem Genet. 2016;54:413–420. doi: 10.1007/s10528-016-9728-y. [DOI] [PubMed] [Google Scholar]
- 28.Zheng W, Zhao J, Tao Y, Guo M, Ya Z, Chen C, Qin N, Zheng J, Luo J, Xu L. MicroRNA-21: A promising biomarker for the prognosis and diagnosis of non-small cell lung cancer. Oncol Lett. 2018;16:2777–2782. doi: 10.3892/ol.2018.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagheri A, Khorram Khorshid HR, Mowla SJ, Mohebbi HA, Mohammadian A, Yaseri M, Solaymani-Dodaran M, Sherafatian M, Tavallaie M. Altered miR-223 expression in sputum for diagnosis of Non-small cell lung cancer. Avicenna J Med Biotechnol. 2017;9:189–195. [PMC free article] [PubMed] [Google Scholar]
- 30.Sromek M, Glogowski M, Chechlinska M, Kulinczak M, Szafron L, Zakrzewska K, Owczarek J, Wisniewski P, Wlodarczyk R, Talarek L, et al. Changes in plasma miR-9, miR-16, miR-205 and miR-486 levels after non-small cell lung cancer resection. Cell Oncol (Dordr) 2017;40:529–536. doi: 10.1007/s13402-017-0334-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang SY, Li Y, Jiang YS, Li RZ. Investigation of serum miR-411 as a diagnosis and prognosis biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21:4092–4097. [PubMed] [Google Scholar]
- 32.Ow SH, Chua PJ, Bay BH. miR-149 as a potential molecular target for cancer. Curr Med Chem. 2018;25:1046–1054. doi: 10.2174/0929867324666170718102738. [DOI] [PubMed] [Google Scholar]
- 33.Kang X, Kong F, Wu S, Liu Q, Yang C, Wu X, Zhang W. microRNA-612 suppresses the malignant development of non-small-cell lung cancer by directly targeting bromodomain-containing protein 4. Onco Targets Ther. 2019;12:4167–4179. doi: 10.2147/OTT.S204004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ling C, Wang X, Zhu J, Tang H, Du W, Zeng Y, Sun L, Huang JA, Liu Z. MicroRNA-4286 promotes cell proliferation, migration, and invasion via PTEN regulation of the PI3K/Akt pathway in non-small cell lung cancer. Cancer Me. 2019;8:3520–3531. doi: 10.1002/cam4.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Zhang L, Wu J, Khadka B, Fang Z, Gu J, Tang B, Xiao R, Pan G, Liu J. CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway. J Exp Clin Cancer Res. 2019;38(54) doi: 10.1186/s13046-019-1071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TY, Choe JW, Pu K, Devulapally R, Bachawal S, Machtaler S, Chowdhury SM, Luong R, Tian L, Khuri-Yakub B, et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J Control Release. 2015;203:99–108. doi: 10.1016/j.jconrel.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin L, Fan Y, Gao F, Jin L, Li D, Sun W, Li F, Qin P, Shi Q, Shi X, Du L. UTMD-promoted Co-delivery of gemcitabine and miR-21 inhibitor by Dendrimer-entrapped gold nanoparticles for pancreatic cancer therapy. Theranostics. 2018;8:1923–1939. doi: 10.7150/thno.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y, Han Z, Shao L, Zhao Y. Evaluation of in vivo antitumor effects of low-frequency ultrasound-mediated miRNA-133a microbubble delivery in breast cancer. Cancer Med. 2016;5:2534–2543. doi: 10.1002/cam4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin D, Li H, Xie H. Ultrasoundtargeted microbubble destructionmediated miR205 enhances cisplatin cytotoxicity in prostate cancer cells. Mol Med Rep. 2018;18:3242–3250. doi: 10.3892/mmr.2018.9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SJ, Lee BR, Kim HS, Ji YR, Sung YH, ShikChoi K, Park HD, Kim SH, Kim MO, Ryoo ZY. Inhibition of migration and invasion by Tet-1 overexpression in human lung carcinoma H460 cells. Oncol Res. 2016;23:89–98. doi: 10.3727/096504015X14496932933539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.