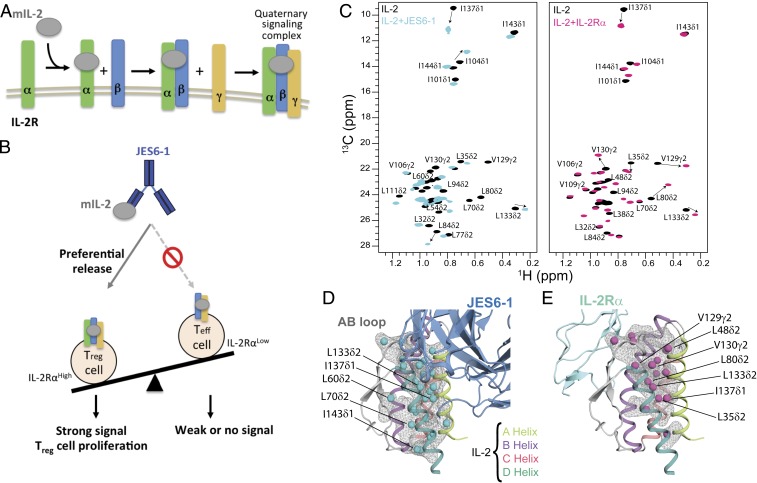
Fig. 1.
A plastic mIL-2 core structure mediates recognition of its binding partners for immune modulation. (A) Schematic of IL-2 cytokine-receptor quaternary complex formation. Assembly of the quaternary complex is thought to occur sequentially, with IL-2 first engaging IL-2Rα with a dissociation constant (Kd) of ∼10−8 M, which increases its affinity for the IL-2Rβ subunit, and finally recruiting the γc subunit to lock down the high-affinity quaternary complex (Kd of ∼10−11 M). (B) Schematic of the proposed mechanism for mIL-2/JES6-1 immunocomplex-mediated selective proliferation of regulatory T (Treg) cells. The JES6-1 Ab (shown as blue scFv) sterically blocks mIL-2 interaction with IL-2Rβ and γc subunits, preventing signaling of IL-2RαLow effector cells (right). However, an exchange mechanism between JES6-1 and the IL-2Rα subunit allows a preferential release of mIL-2 for exclusive signaling on IL-2RαHigh Treg cells, biasing toward an immunosuppressive response (left). (C) Overlay of 1H,13C-HMQC spectra of selectively labeled mIL-2 at the Iδ1-13CH3, L, V proS methyl positions, recorded in the free (black) or as a stoichiometric complex with JES6-1 scFv (cyan) or IL-2Rα receptor (magenta), acquired at 800 MHz, 25 °C. The arrows highlight major chemical shift effects. Selected methyl assignments are noted. (D) Mapping of methyl chemical shift changes on the crystal structure of mIL-2/JES6-1 complex (PDB ID 4YQX) and (E) on the overlaid IL-2Rα subunit from the homologous hIL-2 quaternary complex structure (PDB ID 2B5I). The mIL-2 residues with CSPs > 0.05 ppm are shown with cyan spheres in the schematic representation of JES6-1–bound mIL-2 (blue) and with magenta spheres in IL-2Rα–bound mIL-2 (green).