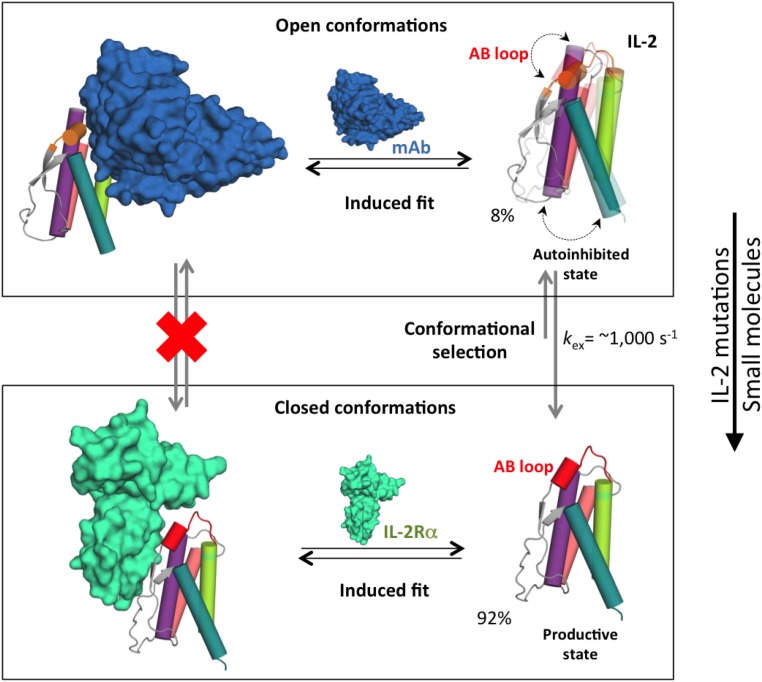
Fig. 6.
Conformational “priming” of free mIL-2 drives high-affinity complex formation. A global conformational transition enables mIL-2 to sample two distinct states that are recognized by IL2-Rα and JES6-1 via additional induced fit steps (black arrows). In the closed conformation, the polar face of the AB loop including R52 is primed to interact with the IL2-Rα receptor. Formation of a C-capping hydrogen bond network by R52 locks the AB loop in an autoinhibitory, open conformation with high affinity for the JES6-1 Ab. As shown in Figs. 3 and 4, point mutations or binding of small molecules can shift the equilibrium to favor the closed conformation, with measurable functional effects (right). Representative exchange parameters and excited-state populations obtained from our NMR data for mIL-2 are indicated. The backbone of the mIL-2 is colored as in Figure 1. Black arrows indicate equilibrium between the free and bound states. Dotted double arrows represent the coupled conformational transition of the AB loop and hydrophobic core of mIL-2. Gray arrows indicate the conformational equilibrium, with the longer arrow pointing to the major state (closed conformation).