Fig. 2.
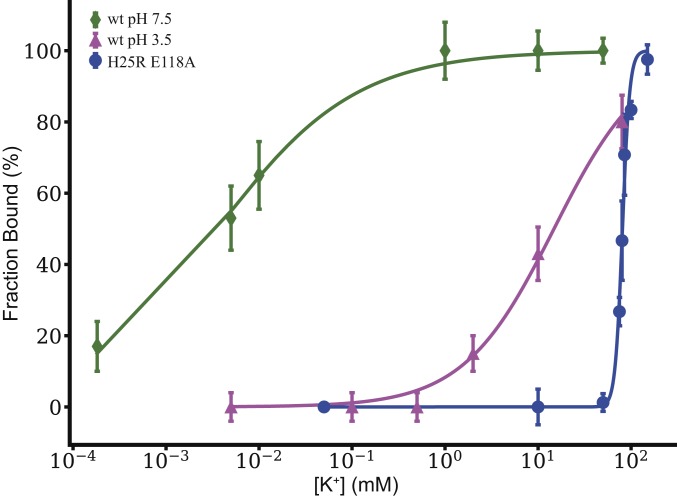
Potassium affinity of the selectivity filter of KcsA based on NMR is presented as a titration plot, presenting the ratio of bound K+ to bound-plus-apo as a function of ambient [K+]. The H25R/E118A mutant (blue) is compared with previously reported data for wild-type pH 7.5 (green) and wild-type pH 3.5 (magenta) (13). Kapp of the open pH gate mutant was calculated to be 81 ± 1 mM by fitting the data to a Hill binding model, compared with 4 ± 1 μM for wild type at pH 7.5 and 14 ± 1 mM for wild type at pH 3.5 (13). These data show that the H25R/E118A mutant with a constitutively open pH gate exhibits a K+ affinity that is somewhat looser still as compared to the open wild-type KcsA at pH 3.5.