Abstract
All movements are generated by the activation of motoneurons, and hence their input-output properties define the final step in processing of all motor commands. A major challenge to understanding this transformation has been the striking nonlinear behavior of motoneurons conferred by the activation of persistent inward currents (PICs) mediated by their voltage-gated Na+ and Ca2+ channels. In this review, we focus on the contribution that these PICs make to motoneuronal discharge and how the nonlinearities they engender impede the construction of a comprehensive model of motor control.
Keywords: motoneuron, persistent inward currents, input-output function, nonlinear behavior
Introduction
To generate sustained, repetitive firing, all neurons in the central nervous system (CNS) require a source of persistent inward current (PIC or Ip) that is resistant to inactivation at depolarized membrane potentials (52). Schwindt and Crill (64) were the first to describe a PIC in mammalian motoneurons, which they subsequently deduced was carried predominantly by calcium ions (65). Subsequently, Hounsgaard and colleagues (23) discovered that motoneuron PICs are greatly facilitated by serotonin and norepinephrine released by axons descending from the brain stem, implying that large PICs might be a standard component of normal motor output. Over the ensuing 40 years, we have learned a great deal about the sources of PICs in motoneurons, the nonlinearities they induce in motoneuronal input-output properties, and their fundamental role in shaping and regulating the motor outflow from the CNS to the musculature during normal motor behavior. It is our intent to provide a short review of this expansive literature, with an emphasis on integrating findings derived from molecular biophysics, cellular neurophysiology, and human motor unit recordings.
Molecular Mechanisms Underlying PICs
In adult motoneurons, both voltage-gated sodium channels (Nav1.1 and 1.6) and L-type voltage-gated calcium channels (Cav1.2 and 1.3) are primary sources of PIC. Both the Nav and L-type Cav channels are widely expressed on the somato-dendritic surfaces of motoneurons. In addition, Nav channels exhibit a particularly dense concentration at the axon initial segment (AIS) that is presumed to be instrumental in action potential initiation (12) and is also likely to contribute to the amplification of synaptic inputs (68). For both families of channels, gating and inactivation properties are modulated by both prior neural discharge and metabotropic inputs (reviewed in Refs. 19, 20, 26, 52). The relative contributions of persistent Na current (INaP) and PICs carried by Ca2+ ions (ICaP) to the total PIC expressed in motoneurons vary among different species, different motoneuron locations (i.e., spinal cord or brain stem), and probably behavioral state (20, 52).
The biophysical properties of Nav and L-type Cav channels have been extensively studied in both patches of membrane excised from neurons (e.g., Ref. 47) and heterologous expression systems (e.g., Ref. 42). Nav channels are protein complexes comprised of one α subunit that forms the pore of the channel responsible for voltage-dependent gating and ion permeation and several auxiliary β subunits (7). The β subunits are small proteins (22–36 kDa) and cross the cell membrane only once. In contrast, the α subunits are much larger proteins (∼260 kDa) composed of four homologous domains (I–IV), with each domain consisting of six α-helical transmembrane-spanning segments (S1–S6). Nine α-subunit isoforms have been discovered to date, four of which are expressed predominantly in adult neurons in the CNS: Nav1.1, Nav1.2, Nav1.3, and Nav1.6 (14). It has recently been shown that Nav1.5 assembles, gates, and functions as a dimer, which may also be the case for the other α-subunit isoforms (10). Ion permeation is blocked by tetrodotoxin in all of the CNS isoforms (7).
In addition to the transient, rapidly inactivating sodium current (INaT) responsible for the upstroke of the action potential (22), Nav channels generate a INaP, which is maintained during long depolarizations and is essential to sustaining repetitive firing (31). The sodium channel α subunit(s) responsible for INaP in motoneurons has not been identified, but it is likely that Nav1.6 is a primary contributor, since it is in cerebellar Purkinje neurons and in cortical pyramidal neurons (43, 59). In addition to Nav1.6, the other four primary brain Na+ channel α-subunit isoforms all can produce INaP. Furthermore, the Cav1.3 L-type Ca2+ channel may also generate some INaP as it appears to do in pacemaker cells of the sinoatrial node (70). The relative contribution of these different channel types to INaP probably varies widely according to cell expression pattern and/or degree of neuromodulation of individual channel subtypes.
Analysis of Nav1.6 channel behavior in a heterologous expression system (HEK-293 cells) revealed that the molecular mechanism of whole-cell INaP is likely to be single-channel delayed opening and re-opening during sustained depolarization (9). Moreover, these manifestations of persistence at the single-channel level increase with the level of depolarization. The mechanisms underlying persistence of INa have not been studied for other Nav isoforms, nor have they been for any native Nav channel in neurons. Furthermore, it is not known whether the mechanisms underlying persistence in Nav1.6 channels described above are modulated by repetitive activation or monoamines.
Studies of modulation of INaP have been directed primarily at the inactivation gate (IG) of the channel. The modulatory mechanisms are mediated by intracellular G-protein signal transduction pathways that are activated by monoamines. INaP is increased when G-protein βγ subunits interact with their binding site on the COOH terminal of the channel (41). Gβγ subunits do not alter the voltage dependence of activation or inactivation of INaT, but shift the voltage dependence of inactivation of INaP positively with respect to that of INaT. It is hypothesized that the Gβγ generates INaP by destabilizing fast inactivation and switching a fraction of sodium channels to a non-inactivating gating mode (41). The molecular basis is hypothesized to be the formation of calcium-calmodulin (CaM) bridges from the COOH-terminal IQ motif to the DIII-IV linker via individual NH2 and COOH lobes of the channel, respectively, that destabilizes binding of the inactivation gate to its receptor, thus biasing inactivation toward more depolarized potentials (42, 62).
A second putative mechanism of modulation has recently been revealed from studies of the Nav1.5 isoform that is expressed primarily in cardiac cells (28). Calmodulin binds to two sites in the intracellular loop that constitutes the IG in a Ca2+-dependent manner that promotes recovery from inactivation while impeding the kinetics of inactivation (28). It is presently unknown whether an analogous mechanism might be operating on other Nav channel isoforms in neurons.
PICs carried by Ca2+ ions (ICaP) are mediated by voltage-gated L-type Ca2+ channels that are composed of a pore-forming α1 subunit and four additional accessory subunits: α2, β, γ, δ (8). Four different α1 subunits have been identified to date, referred to as Cav1.1, Cav1.2, Cav1.3, and Cav1.4. The “L-type” refers to the long activation time of the channels, which are also called dihydropyridine (DHP) channels, since they are blocked by DHPs like nifidipine and nimodipine. Cav1.2 and Cav1.3 channels are predominantly responsible for ICaP in neurons (40). Cav1.3 activates at membrane potentials that are ~10–20 mV more negative than those for Cav1.2 channels (39). As is the case for Nav channels described above, the pore-forming α1 subunit is a protein of ~2,000 amino-acid residues organized in four repeated domains (I–IV), each of which contains six transmembrane segments (S1–S6), and a membrane-associated loop between transmembrane segments S5 and S6. The intracellular β subunit has no transmembrane segments, whereas the γ subunit is a glycoprotein with four transmembrane segments. Four distinct genes encode L-type Cav channel β-subunits, each with multiple splice variants. Alternative splicing creates the short COOH-terminus variant of CaV1.3 (i.e., CaV1.3S), which activates at even more negative potentials than the full-length (long) COOH-terminus variant (i.e., CaV1.3L) (77). Splice variation may also affect the activation voltage range of CaV 1.2 channels, but only a few have been functionally studied so far (38). In addition, four α2δ genes have been identified. Cell-specific combinations of these subunits confer a wide range of distinct functional properties on these channels (8).
ICaP is profoundly modulated by several intracellular signal cascades that are triggered by the activation of metabotropic receptors on the cell membrane of neurons. Activation of 5-HT, mGluRI, and muscarinic receptors by monoamines modulate ICaP in motoneurons through the PLC, IP3 cascade leading to release of Ca2+ from intracellular stores (45), and ICaP is enhanced through the β2-adrenergic receptor (β2AR)-cAMP-PKA cascade (58).
The function of CaV1.2 and 1.3 channels is tightly regulated by changes in intracellular Ca2+ concentration ([Ca2+]i) and the subsequent binding of Ca2+ to several sites on the intracellular COOH-terminal tails of the channels. Both the opening of CaV channels and the release of Ca2+ from intracellular stores cause a local increase in Ca2+ concentration that induces two opposing regulatory mechanisms: Ca2+-dependent inactivation (CDI) and Ca2+-dependent facilitation (CDF) (1). CDF manifests in neurons as an increase in the magnitude of the ICaP with repetitive activation (47, 51, 53), which is thought to be mediated by Ca2+-/CaM-dependent kinase II (CaMKII)-mediated phosphorylation and Ca2+/CaM binding in the COOH-terminal tail of these channels (24).
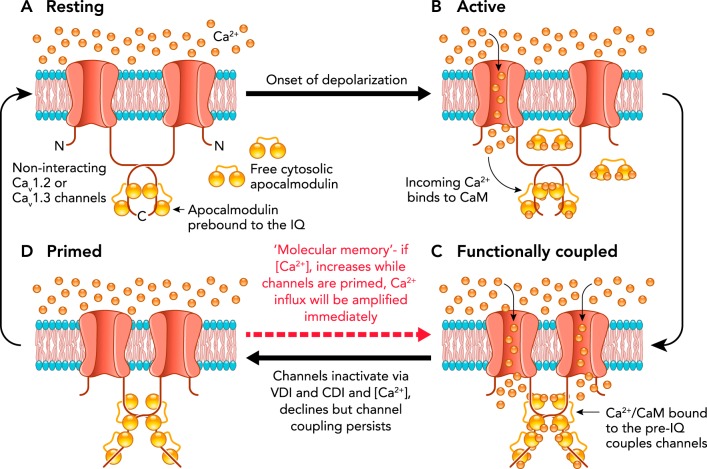
An additional mechanism mediating facilitation of ICaP has recently been described for both CaV1.2 and the short-tailed isoform of CaV1.3 channels (i.e., CaV1.3S). A combination of super-resolution imaging, optogenetics, and electrophysiological measures revealed that both CaV1.2 and the short-tailed isoform of CaV1.3 channels (i.e., CaV1.3S) associate in functional clusters (11, 46), formed by stochastic self-assembly (63), within which adjacent channels open cooperatively, facilitating Ca2+ influx. The cooperative channels are coupled via a COOH terminus-to-COOH terminus allosteric interaction that requires binding of the incoming Ca2+ to calmodulin (CaM) and subsequent binding of CaM to the pre-IQ domain of the channels. Functionally coupled channels facilitate ICaP as a consequence of their higher open probabilities (11, 46), as illustrated in FIGURE 1. Moreover, the coupled channels can open at membrane potentials well below their normal activation threshold as long as local calcium remains elevated. Thus, in effect, the L-type Ca2+ channels are both voltage-gated and calcium-gated. The mechanism by which the transient binding of adjacent channels increases their open probability is unknown at present but is likely to result from the tension exerted on the channel pore lining consequent to COOH-terminal dimerization. Regardless of the mechanism, the increase in L-type Ca2+ channel open probability (Po) caused by channel coupling may contribute to the relatively slow PIC activation observed in situ (33, 34) as well as PIC facilitation by repeated activation.
FIGURE 1.
Proposed model of the functional coupling of L-type calcium channels
A: CaV1 channels are arranged in dense clusters in the membranes of excitable cells. For simplicity, a cluster of two channels is shown above. At the resting membrane potential (e.g., −70 mV), [Ca2+]i and CaV1 channel open probabilities (Po) are very low. B: with membrane depolarization, some CaV1 channels open, producing an elevation in local [Ca2+]i and increasing Ca2+ binding to calmodulin (CaM). C: Ca2+CaM binding to the COOH-terminal pre-IQ domain of the CaV1 channel promotes physical interactions between adjacent channels within a cluster. This functional coupling increases the open probabilities of the adjoined channels and thus amplifies Ca2+ influx. D: CaV1 channels undergo VDI and CDI, and [Ca]i declines once more. However, the channels remain coupled in a “primed,” non-conducting state for a finite time. If the membrane is depolarized again when the channels are still primed, the amplification of Ca2+ influx will be immediate; otherwise, if [Ca2+]i remains at resting levels beyond the lifetime of the primed state (~1 s), the coupling dissolves and the cycle begins again. Modified from Refs. 11, 46, with permission from eLife.
Effects of PICs on Motoneuron Discharge
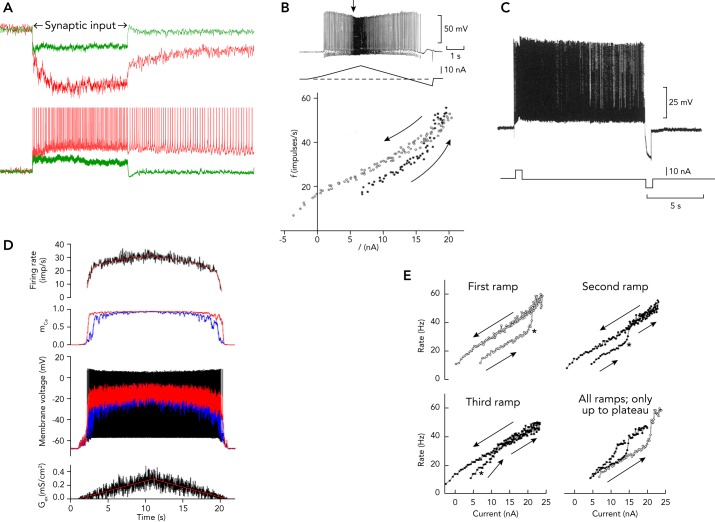
PICs make several key contributions to the behavior of motoneurons, as illustrated in FIGURE 2 and summarized in Table 1. INaP enables motoneurons to generate repetitive firing in the presence of a sustained, depolarizing synaptic drive (18, 31) (FIGURE 2, A–E). In addition, the combined contributions of INaP and ICaP create PICs that can generate a three- to fivefold amplification of the currents that the motoneurons receive from their synaptic inputs. The extent of this amplification depends on the level of monoaminergic drive (25, 32) (compare red and green traces in FIGURE 2A). Amplification of synaptic inputs is essential for generating the levels of depolarizing drive to the motoneurons required to produce their optimal discharge rates (5). Although INaP undergoes slow inactivation within a few seconds, ICaP exhibits little inactivation (36, 37, 47, 50), and thus PICs can sustain repetitive discharge in some motoneurons long after the cessation of the synaptic input that initiates the firing (FIGURE 2, A AND C) (23, 34). This phenomenon reflects the fact that a strong PIC leads to an N-shaped steady-state current-voltage relation that has two stable membrane states: one at the resting potential and one at a more depolarized level (17). Depolarizing input pushes the membrane to the more depolarized state, where it can remain until pushed back toward the resting state by hyperpolarizing current. The firing rate hysteresis in response to triangular ascending and descending inputs (FIGURE 2B) is attributable to the same mechanism. Persistent, self-sustained motoneuron discharge can be abruptly truncated by activating an inhibitory input (FIGURE 2C) (23). Thus hysteresis in response to both a triangular input and self-sustained firing are expressions of the PICs.
FIGURE 2.
Effects of PICs on motoneuron discharge recoded in experimental animals
A: amplification of synaptic currents generated by Ia afferent fibers and self-sustained firing in a cat spinal motoneuron (based on Refs. 33, 34). B: firing rate hysteresis in response to ascending and descending inputs to a motoneuron (from Fig. 5 of Ref. 23). C: production of repetitive firing in a spinal motoneuron by a depolarizing current pulse and termination of firing by a hyperpolarizing current pulse (from Fig. 6 in Ref. 23). D: saturation of motoneuron discharge induced by fully activated PIC (56). E: facilitation or warm-up of motor unit discharge consequent to repetitive triangular, injected current waveforms (2). Images are from Refs. 2, 56 are used with permission from Journal of Neurophysiology; images from Ref. 23 are used with permission from Journal of Physiology.
Table 1.
Effects of persistent inward currents on motoneuron discharge properties
| PIC Effect | Effect on Motoneuron Discharge | Biophysical Mechanism | Putative Function |
|---|---|---|---|
| Amplification of synaptic inputs | Produces an initial acceleration in firing rate as the PIC activates. Lasts ~1–2 s. | Gradual voltage-dependent activation of INap and ICaP. The relatively slow acceleration may reflect increasing CDF of ICaP. | Amplification, with the level proportional to monoaminergic drive. Potentially provides gain control. |
| Saturation | Decreased sensitivity to increases in input currents. | Activation of Cav1.2 and 1.3s channels that depolarize the dendritic tree to near the reversal potential for excitatory inputs. | Unknown; perhaps a necessary consequence of amplification. The potency of inhibitory input is increased during saturation. |
| Hysteresis | De-recruitment occurs at a lower input level than recruitment. | Mainly due to ICaP and membrane bistability resulting from an N-shaped I-V relation. Requires a strong PIC that is maintained by CDF. | Self-sustained firing appears to be the foundation of steady output for posture and stabilization tasks. |
| Facilitation | Increased firing rate in response to repeated inputs. | CDF and cooperative gating of Cav1.2 and 1.3s channels. | Unknown. |
PIC, persistent inward current; CDF, calcium-dependent facilitation; INap, peristent Na current; ICaP, PIC carried by Ca2+ ions.
In addition to the amplification and hysteretic/self-sustained firing, there are two additional effects of PICs on motoneuron firing rate: saturation and facilitation or “warm-up.” Firing rate saturation occurs when the PIC is fully activated, creates a stable firing-rate plateau, and renders the motoneurons less sensitive to further increases in excitatory input. FIGURE 2D shows the effects of a simulated, noisy excitatory conductance input (bottom) on firing rate (top), PIC activation (second panel), and membrane potential in the somatic and dendritic compartments (third panel) of a motoneuron model. The firing rate saturates when the dendritic compartments are depolarized to near the excitatory synaptic reversal potential (32, 54). This saturation occurs at a much lower input level during natural synaptic activation than during injected current, because both the post-synaptic receptors and the PIC-generating channels are located primarily on the dendrites (3, 32) that constitute roughly 95% of the total motoneuron membrane surface area (72). This differential impact is clear from comparison of FIGURE 2D (simulated synaptic input, strong saturation) with FIGURE 2, B AND E (injected currents, minimal saturation).
Facilitation or warm-up are terms used to describe the progressive increase in the PIC amplitude with repetitive activation (69) that leads to increased firing rate hysteresis and earlier firing rate acceleration (FIGURE 2E). The underlying mechanism is likely to be the CaM-dependent facilitation of Cav1.2 and Cav1.3s channels that leads to an increase in their open probabilities (11, 46, 47, 51), as described in the previous section Molecular Mechanisms Underlying PICs and illustrated in FIGURE 1.
The overall impact of PICs on motoneuron behavior is to transform these cells from linear transducers of synaptic or injected currents (49) into highly nonlinear actuators, whose behaviors are critically dependent on prior activity and neuromodulatory state. The self-sustained discharge, discharge hysteresis, firing rate acceleration, firing rate saturation, and warm-up effects described in this section, illustrated in FIGURE 2, and summarized in Table 1 are all consequences of the nonlinearities attributable to PIC activation.
The Role of PICs in Regulating Motor Output in Humans
Our understanding of how PICs regulate normal motor output in man is based on inferences drawn from studying the firing patterns of human motor units under experimental conditions where PICs are likely to be activated (19, 26, 29). As discussed in the previous section, three important effects of PICs on synaptic input uncovered in animal studies are amplification, saturation, and hysteresis. These effects are illustrated in FIGURE 3A for a cat motoneuron, in response to a slowly injected, triangular current. This type of input provides a reasonable match to the time course of the triangular torque patterns often generated by human subjects during studies of their motor unit firing rates (see below). Activation of the PIC results in a dramatic amplification of the depolarizing drive and induces a strong acceleration in the motoneuron’s firing rate (3, 23, 34). Although the onset of the PIC amplification is relatively rapid (1–2 s), it is slow compared with the kinetics of most voltage-gated ion channels (1–10 ms; see Ref. 7). This latency may reflect, in part, the time required to enhance the opening of Cav1.2 and Cav1.3s channels through CaM-dependent facilitation (11, 46). Once fully activated, the PIC makes the cell insensitive to further increases in excitatory input, resulting in a saturation or plateau in the cell’s firing rate (25, 32, 33). As described earlier and illustrated in FIGURE 1, Cav1.2 and Cav1.3s channels sustain their facilitated state as long as the local Ca2+ concentration remains elevated (11, 46), which prolongs the PIC and renders the motoneurons resistant to deactivation (47). Thus the firing-rate profile displays a striking hysteresis, in that the offset of firing (de-recruitment) occurs at a lower input level than its onset (recruitment). Facilitation and prolongation of the PIC is also manifest as self-sustained firing after a relatively brief period of excitatory input (23) (FIGURE 2, A AND C). In contrast, the firing patterns in response to this same input in animal preparations with low levels of monoamines to inhibit the activation of PICs (red trace in FIGURE 3A) exhibit neither acceleration nor saturation, and the hysteresis in firing rate has the opposite pattern (offset at higher level than onset) (4, 23, 35, 44). Not shown in this figure is the consistent finding that an inhibitory input results in the abrupt cessation of the PIC (as seen in FIGURE 2C).
FIGURE 3.
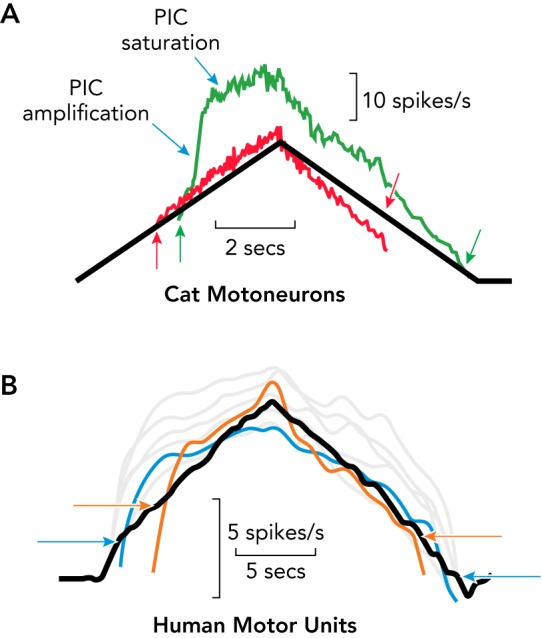
Comparison of the effects of PIC on cat motoneuron discharge
Comparison of the effects of PICs on cat motoneuron discharge (A) (modified from (29)) and their presumed effects on human motor unit discharge (B) (Heckman CJ, Thompson CK, unpublished data). A: the red trace shows a firing pattern in response to a triangular-shaped pattern of injected current (black trace) in a cat motoneuron lacking a strong PIC. The green trace was recorded in response to the same input from a cat motoneuron with a strong PIC. Note the acceleration in firing induced by the rapid amplification of the PIC, followed by saturation. The arrows at onset and offset indicate the hysteresis (offset at lower input level than onset for the PIC motoneuron). B: the firing patterns from seven human motor units recorded during a volitionally generated, torque output in isometric conditions. The similarity between the cat and human data is striking. The blue and orange traces have arrows to indicate the onset-offset hysteresis. Images from Ref. 29 are used with permission from Journal of Neurophysiology.
FIGURE 3B displays the firing patterns of seven motor units simultaneously recorded from the tibialis anterior muscle of a human subject (two traces are in color to emphasize the pattern; the rest are gray) in using a triangular torque protocol designed to match the injected current triangles used in cat motoneuron experiments (FIGURE 3A). The expressions of the PICs observed in the intracellular recording (amplification, saturation, hysteresis; green trace in FIGURE 3A) are clearly present in the human motor unit firing patterns. Furthermore, self-sustained firing of motor units in human subjects generated in response to even a brief input has been demonstrated in several studies (16, 30, 74). In addition to these close similarities between PIC-induced behavior in cat motoneuron firing patterns and those observed in human motor unit recordings, Fuglevand and colleagues (13, 61) provided strong evidence that the acceleration in firing rate in human motor units is consequent to the rapid onset of PIC amplification and that firing rate saturation is due to PIC depolarization of the dendrites. The addition of a steady background of inhibition, which is known to sharply reduce PIC amplitude in cat motoneurons (27), eliminates the acceleration in firing rate in human motor units (61). Equally striking, once a motor unit’s firing rate reaches its saturation level, it becomes insensitive to additional excitatory input, as is the case for cat motoneurons (27).
Gorassini and colleagues (13) have effectively used the type of hysteresis illustrated in FIGURE 3B to estimate the magnitude of the PICs in human subjects by comparing the firing patterns of pairs of simultaneously active motor units. The firing frequency of the lower-threshold motor unit of a pair provides an estimate of the synaptic drive to the motoneuron pool as the higher-threshold motor unit is recruited and de-recruited. The difference in firing frequency of the lower-threshold unit when the higher threshold unit is recruited and de-recruited reflects the hysteresis in the PIC and is referred to as ΔF. Because ΔF is proportional to the amplitude of the PIC (55), it also provides an index of the strength of monoaminergic input from the brain stem. The factors influencing the accuracy of the ΔF metric in humans have been studied (57, 66), and the validity of the ΔF method has been tested by intracellular measurements of PICs (15) and computer simulations of motoneuron input-output functions (55, 60).
A number of studies using the ΔF method have established that PICs make a prominent contribution to motor unit discharge in human muscles throughout the arm, trunk, and leg (e.g., Refs. 48, 67, 71, 73, 76). There are, however, rather striking variations in strength of the PIC in different muscles. For example, Wilson and colleagues (70) showed that ΔF is nearly twice as large in the triceps muscles as it is in biceps. Similarly, PICs in the human ankle flexor, tibialis anterior, are considerably larger than those in the ankle extensor, soleus (Ref. 13; Heckman CJ, et al., unpublished observations). It is likely that a systematic analysis of ΔF in multiple muscles across the human body will be needed to fully appreciate the functional roles of these differences in PICs.
From the perspective of functional significance in normal motor behavior, two effects of PICs in motoneurons, amplification of synaptic inputs and self-sustained firing, are particularly important. From the initial studies of PICs in cat motoneurons, it was postulated that self-sustained firing is instrumental for generating the sustained force outputs required for postural control (23). The much greater tendency of low-threshold motoneurons to exhibit self-sustained firing (33, 34) supports this hypothesis. PIC amplification appears to be essential for generating all but the lowest levels of motor output. Measurements of the synaptic currents generated by both peripheral and descending inputs in animal experiments reveal that they provide insufficient depolarizing drive to generate high motoneuron discharge rates required to produce strong muscle activation (52). The potent PIC amplification of synaptic inputs and its proportional scaling via descending monoaminergic input may provide a means for regulating the input-output gain of the somatic motor system: low input-output gains are beneficial for generating low-force, precise movements, whereas progressively higher gains are needed for more forceful motor behaviors (6, 75). Results consistent with this “gain control hypothesis” have been obtained in human subjects (75). In addition, studies of neurons in the motor cortex during increasing effort in primates also support the existence of this gain control mechanism (49).
Although PIC amplification of synaptic inputs is essential, its rapid activation to produce firing-rate acceleration and its induction of firing-rate saturation have no obvious function. This firing-rate acceleration/saturation produces a striking nonlinearity in input-output behavior of individual motor units, and computer simulations demonstrate that this nonlinearity distorts the input-output function for the motor pool as a whole. FIGURE 4 is based on computer simulations that are closely tuned to replicate the effects of PICs on cat motoneuron firing patterns (56), which, as illustrated in FIGURE 3A, closely replicate human motor unit firing patterns (29). A linearly increasing and decreasing synaptic conductance was applied equally to all motoneurons in the simulated pool. Each trace in this figure represents the averaged firing rate of the entire pool and thus reflects the full pattern of recruitment and rate modulation of all simulated motoneurons. As the PIC amplitudes were increased (blue to red), the output became less and less linear due to increasing firing-rate acceleration and saturation (see FIGURE 3A). Perhaps these strong PIC-induced nonlinearities represent a modest trade-off for the huge advantages of PIC amplification and self-sustained firing.
FIGURE 4.
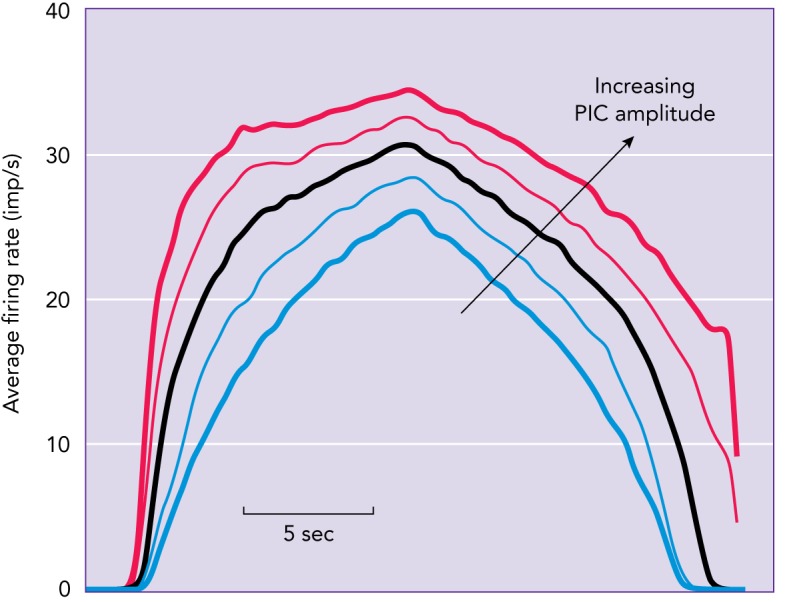
Computer simulations of the effect of the PIC on motor unit firing patterns
A linearly increasing and decreasing synaptic conductance was applied equally to all motoneurons in the simulated pool, and each trace represents the averaged firing rate of the entire pool and thus reflects the full pattern of recruitment and rate modulation of all simulated motoneurons. As PIC amplitude increases (bottom to top), nonlinearity increases. Images are from Ref. 56 and used with permission from Journal of Neurophysiology.
Finally, it has been speculated that motor commands use excitation and inhibition in concert with the descending monoaminergic drive to achieve flexible control of PICs. This might enable the motor system to match motoneuron excitability to the requisite motor output over the wide range of motor behaviors we observe in animals, including man (21, 29). For example, coupling inhibition and excitation in a push-pull fashion (a form of reciprocal inhibition in which a baseline of inhibition is decreased as excitation is increased) may provide effective control of the PIC, allowing amplification of synaptic inputs without the prolongation of the PIC (26, 27). The mechanisms by which the precise matching of excitation, inhibition, and neuromodulation required to generate complex, controlled motor behaviors are achieved, however, remain elusive and undiscovered.
Acknowledgments
Work on this review was supported by National Institute of Neurological Disorders and Stroke Grants NS-089313, NS-098509, and NS-110953.
No conflicts of interest, financial or otherwise, are declared by the author(s).
M.B., R.K.P., and C.H. conceived and designed research; M.B., R.K.P., and C.H. performed experiments; M.B., R.K.P., and C.H. analyzed data; M.B., R.K.P., and C.H. interpreted results of experiments; M.B., R.K.P., and C.H. prepared figures; M.B., R.K.P., and C.H. drafted manuscript; M.B., R.K.P., and C.H. edited and revised manuscript; M.B., R.K.P., and C.H. approved final version of manuscript.
References
- 1.Ben-Johny M, Yue DT. Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J Gen Physiol 143: 679–692, 2014. doi: 10.1085/jgp.201311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2038–2045, 1998. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- 5.Binder MD. Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev 40: 1–8, 2002. doi: 10.1016/S0165-0173(02)00183-2. [DOI] [PubMed] [Google Scholar]
- 6.Binder MD, Stuart DG. Motor unit-muscle receptor interactions: design features of the neuromuscular control system. In: Spinal and Supraspinal Mechanisms of Voluntary Motor Control and Locomotion, edited by Desmedt JE. Basel: Karger, 1981, p. 72–98. [Google Scholar]
- 7.Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem 64: 493–531, 1995. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555, 2000. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 9.Chatelier A, Zhao J, Bois P, Chahine M. Biophysical characterisation of the persistent sodium current of the Nav1.6 neuronal sodium channel: a single-channel analysis. Pflugers Arch 460: 77–86, 2010. doi: 10.1007/s00424-010-0801-9. [DOI] [PubMed] [Google Scholar]
- 10.Clatot J, Hoshi M, Wan X, Liu H, Jain A, Shinlapawittayatorn K, Marionneau C, Ficker E, Ha T, Deschênes I. Voltage-gated sodium channels assemble and gate as dimers. Nat Commun 8: 2077, 2017. doi: 10.1038/s41467-017-02262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon RE, Moreno CM, Yuan C, Opitz-Araya X, Binder MD, Navedo MF, Santana LF. Graded Ca2+/calmodulin-dependent coupling of voltage-gated CaV1.2 channels. eLife 4: e05608, 2015. doi: 10.7554/eLife.05608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duflocq A, Chareyre F, Giovannini M, Couraud F, Davenne M. Characterization of the axon initial segment (AIS) of motor neurons and identification of a para-AIS and a juxtapara-AIS, organized by protein 4.1B. BMC Biol 9: 66, 2011. doi: 10.1186/1741-7007-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuglevand AJ, Lester RA, Johns RK. Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. J Neurophysiol 113: 1310–1322, 2015. doi: 10.1152/jn.00777.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron 28: 365–368, 2000. doi: 10.1016/S0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 15.Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- 16.Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998. doi: 10.1016/S0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- 17.Gutman AM. Bistability of dendrites. Int J Neural Syst 01: 291–304, 1991. doi: 10.1142/S0129065791000339. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heckman CJ, Enoka RM. Motor unit. Compr Physiol 2: 2629–2682, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Heckmann CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- 21.Heckman CJ, Johnson MD. Reconfiguration of the electrical properties of motoneurons to match the diverse demands of motor behavior. Adv Exp Med Biol 826: 33–40, 2014. doi: 10.1007/978-1-4939-1338-1_3. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544, 1952. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol 171: 537–547, 2005. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552: 945–952, 2003. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hultborn H, Zhang M, Meehan CF. Control and role of plateau potential properties in the spinal cord. Curr Pharm Des 19: 4357–4370, 2013. doi: 10.2174/1381612811319240004. [DOI] [PubMed] [Google Scholar]
- 27.Hyngstrom AS, Johnson MD, Heckman CJ. Summation of excitatory and inhibitory synaptic inputs by motoneurons with highly active dendrites. J Neurophysiol 99: 1643–1652, 2008. doi: 10.1152/jn.01253.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson CN, Potet F, Thompson MK, Kroncke BM, Glazer AM, Voehler MW, Knollmann BC, George AL, Jr., Chazin WJ. A mechanism of calmodulin modulation of the human cardiac sodium channel. Structure 26: 683–294e683, 2018. doi: 10.1016/j.str.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson MD, Thompson CK, Tysseling VM, Powers RK, Heckman CJ. The potential for understanding the synaptic organization of human motor commands via the firing patterns of motoneurons. J Neurophysiol 118: 520–531, 2017. doi: 10.1152/jn.00018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- 31.Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006. doi: 10.1113/jphysiol.2006.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- 34.Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- 35.Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- 36.Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol 82: 2518–2527, 1999. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- 38.Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem 282: 35133–35142, 2007. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- 39.Lieb A, Ortner N, Striessnig J. C-terminal modulatory domain controls coupling of voltage-sensing to pore opening in Cav1.3 L-type Ca(2+) channels. Biophys J 106: 1467–1475, 2014. doi: 10.1016/j.bpj.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol 92: 2633–2641, 2004. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 41.Ma JY, Catterall WA, Scheuer T. Persistent sodium currents through brain sodium channels induced by G protein betagamma subunits. Neuron 19: 443–452, 1997. doi: 10.1016/S0896-6273(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 42.Mantegazza M, Yu FH, Powell AJ, Clare JJ, Catterall WA, Scheuer T. Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits. J Neurosci 25: 3341–3349, 2005. doi: 10.1523/JNEUROSCI.0104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci 21: 2268–2277, 2001. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meehan CF, Sukiasyan N, Zhang M, Nielsen JB, Hultborn H. Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. J Neurophysiol 103: 2599–2610, 2010. doi: 10.1152/jn.00668.2009. [DOI] [PubMed] [Google Scholar]
- 45.Mejia-Gervacio S, Hounsgaard J, Diaz-Muñoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience 123: 123–130, 2004. doi: 10.1016/j.neuroscience.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Moreno CM, Dixon RE, Tajada S, Yuan C, Opitz-Araya X, Binder MD, Santana LF. Ca(2+) entry into neurons is facilitated by cooperative gating of clustered CaV1.3 channels. eLife 5: e15744, 2016. doi: 10.7554/eLife.15744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol 98: 1042–1047, 2007. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- 48.Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009. doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naufel S, Glaser JI, Kording KP, Perreault EJ, Miller LE. A muscle-activity-dependent gain between motor cortex and EMG. J Neurophysiol 121: 61–73, 2019. doi: 10.1152/jn.00329.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89: 954–959, 2003. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- 51.Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528: 107–113, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- 53.Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol 83: 483–500, 2000. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- 54.Powers RK, Elbasiouny SM, Rymer WZ, Heckman CJ. Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: a simulation study. J Neurophysiol 107: 808–823, 2012. doi: 10.1152/jn.00510.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Powers RK, Heckman CJ. Contribution of intrinsic motoneuron properties to discharge hysteresis and its estimation based on paired motor unit recordings: a simulation study. J Neurophysiol 114: 184–198, 2015. doi: 10.1152/jn.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powers RK, Heckman CJ. Synaptic control of the shape of the motoneuron pool input-output function. J Neurophysiol 117: 1171–1184, 2017. doi: 10.1152/jn.00850.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian H, Patriarchi T, Price JL, Matt L, Lee B, Nieves-Cintrón M, Buonarati OR, Chowdhury D, Nanou E, Nystoriak MA, Catterall WA, Poomvanicha M, Hofmann F, Navedo MF, Hell JW. Phosphorylation of Ser1928 mediates the enhanced activity of the L-type Ca2+ channel Cav1.2 by the β2-adrenergic receptor in neurons. Sci Signal 10: eaaf9659, 2017. doi: 10.1126/scisignal.aaf9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526, 1997. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Revill AL, Fuglevand AJ. Effects of persistent inward currents, accommodation, and adaptation on motor unit behavior: a simulation study. J Neurophysiol 106: 1467–1479, 2011. doi: 10.1152/jn.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Revill AL, Fuglevand AJ. Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents. J Physiol 595: 179–191, 2017. doi: 10.1113/JP272823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarhan MF, Tung CC, Van Petegem F, Ahern CA. Crystallographic basis for calcium regulation of sodium channels. Proc Natl Acad Sci USA 109: 3558–3563, 2012. doi: 10.1073/pnas.1114748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato D, Hernández-Hernández G, Matsumoto C, Tajada S, Moreno CM, Dixon RE, O’Dwyer S, Navedo MF, Trimmer JS, Clancy CE, Binder MD, Santana LF. A stochastic model of ion channel cluster formation in the plasma membrane. J Gen Physiol 151: 1116–1134, 2019. doi: 10.1085/jgp.201912327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res 120: 173–178, 1977. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- 65.Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol 43: 1700–1724, 1980. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- 66.Stephenson JL, Maluf KS. Dependence of the paired motor unit analysis on motor unit discharge characteristics in the human tibialis anterior muscle. J Neurosci Methods 198: 84–92, 2011. doi: 10.1016/j.jneumeth.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephenson JL, Maluf KS. Discharge behaviors of trapezius motor units during exposure to low and high levels of acute psychosocial stress. J Clin Neurophysiol 27: 52–61, 2010. doi: 10.1097/WNP.0b013e3181cb81d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron 15: 1065–1076, 1995. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 69.Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol 78: 1740–1742, 1997. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- 70.Toyoda F, Mesirca P, Dubel S, Ding WG, Striessnig J, Mangoni ME, Matsuura H. CaV1.3 L-type Ca2+ channel contributes to the heartbeat by generating a dihydropyridine-sensitive persistent Na+ current. Sci Rep 7: 7869, 2017. doi: 10.1038/s41598-017-08191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Udina E, D’Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103: 1295–1303, 2010. doi: 10.1152/jn.00734.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulfhake B, Kellerth JO. A quantitative morphological study of HRP-labelled cat alpha-motoneurones supplying different hindlimb muscles. Brain Res 264: 1–19, 1983. doi: 10.1016/0006-8993(83)91116-2. [DOI] [PubMed] [Google Scholar]
- 73.Vandenberk MS, Kalmar JM. An evaluation of paired motor unit estimates of persistent inward current in human motoneurons. J Neurophysiol 111: 1877–1884, 2014. doi: 10.1152/jn.00469.2013. [DOI] [PubMed] [Google Scholar]
- 74.Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol 545: 671–679, 2002. doi: 10.1113/jphysiol.2002.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ, Kording KP. Serotonin affects movement gain control in the spinal cord. J Neurosci 34: 12690–12700, 2014. doi: 10.1523/JNEUROSCI.1855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson JM, Thompson CK, Miller LC, Heckman CJ. Intrinsic excitability of human motoneurons in biceps brachii versus triceps brachii. J Neurophysiol 113: 3692–3699, 2015. doi: 10.1152/jn.00960.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol Rev 67: 821–870, 2015. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]