Figure EV1. Quantification of murine‐ and human‐specific TGF‐β1 in wounds or in TGF‐β1‐siRNA‐transfected MSCs.
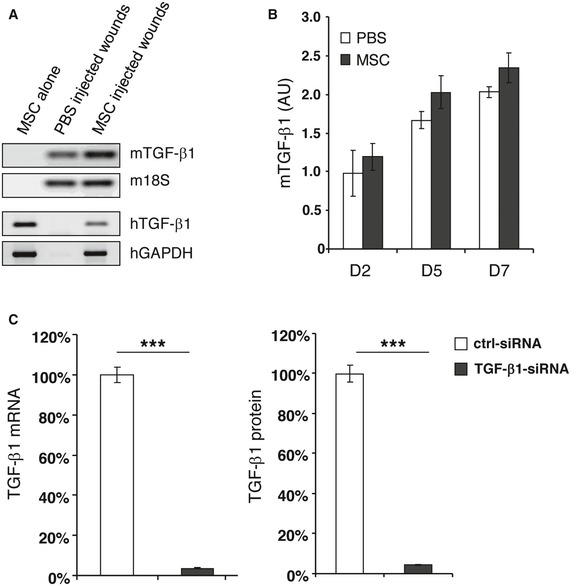
- Total RNA was prepared from cultured AT‐MSCs, or day 2 wound tissues from PBS‐ or AT‐MSC‐injected CD18−/− wounds. Reverse transcription PCR was performed by using primers that amplify murine‐ or human‐specific TGF‐β1. Murine 18S or human GAPDH was used as endogenous control, respectively.
- The expression of murine‐specific TGF‐β1 in PBS‐ or AT‐MSC‐injected CD18−/− wounds at days 2, 5, and 7 post‐wounding was quantified by qPCR using primers that amplify murine‐specific TGF‐β1, and normalized on the expression of murine 18S. Data are given as mean ± SD of triplicate measurements. This experiment was performed three times with similar results. AU, arbitrary unit.
- AT‐MSCs were transfected with TGF‐β1‐specific siRNA targeting four different sites on human TGF‐β1 mRNA, and a control siRNA. 48 h after transfection, the cells and supernatants were harvested. The expression of TGF‐β1 at mRNA level was quantified by qPCR with primers amplify human‐specific TGF‐β1. The release of TGF‐β1 protein in supernatants was measured by TGF‐β1‐specific ELISA. The expression and release of TGF‐β1 in control siRNA‐transfected AT‐MSCs was normalized as 100%. The depicted data of TGF‐β1‐siRNA‐transfected AT‐MSCs were from the siRNA that showed best knockdown effect among the tested four. This TGF‐β1 siRNA was selected for the subsequent experiments. Data are given as mean ± SD of triplicate measurements. ***P < 0.001 by two‐tailed unpaired t‐test.
