Figure 4. TGF‐β1 released by MSCs is responsible for accelerated healing in CD18−/− wounds.
-
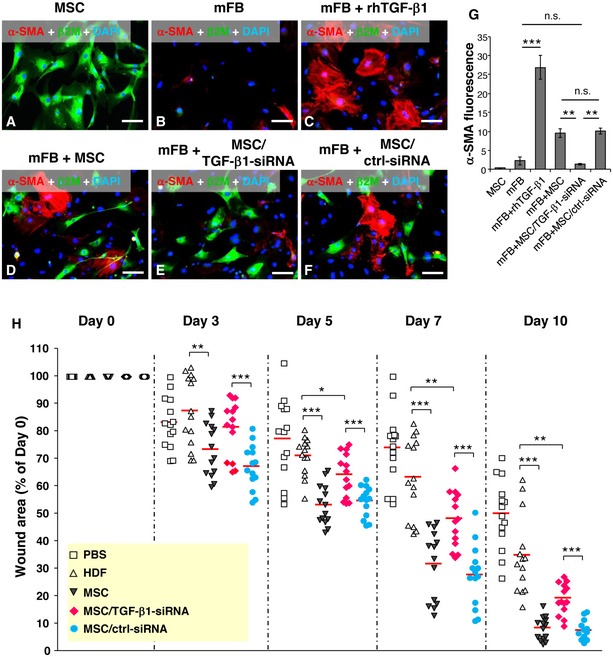
A–FRepresentative photographs of immunofluorescence staining for α‐SMA (red) and human β2M (green) on cultured human AT‐MSCs (A), murine primary dermal fibroblasts (mFB) (B), mFB treated with 2 ng/ml recombinant human TGF‐β1 (C), and cocultures of mFB with AT‐MSCs (D) or TGF‐β1 siRNA‐transfected AT‐MSCs (E) or control siRNA‐transfected AT‐MSCs (F) after 48‐h culture. Nuclei were counterstained with DAPI (blue). Scale bars: 100 μm.
-
GQuantification of α‐SMA immunofluorescence shown in (A–F), mean ± SEM, n ≥ 3 wells per group, **P < 0.01; ***P < 0.001; n.s., not significant, by one‐way ANOVA with Tukey's test.
-
HFour full‐thickness excisional wounds were produced on each of CD18−/− mouse by 6‐mm biopsy punches. Each wound received intradermal injection of 2.5 × 105 AT‐MSCs or human dermal fibroblasts (HDFs) or TGF‐β1 siRNA‐transfected AT‐MSCs or control siRNA‐transfected AT‐MSCs or PBS. Each wound region was digitally photographed at days 0, 3, 5, 7, and 10 post‐wounding, and wound areas were analyzed using Adobe Photoshop. The depicted result is the quantitative analysis of all wound areas per group, expressed as percentage of the initial wound size at day 0. The line in each group represents the mean value. *P < 0.05, **P < 0.01, ***P < 0.001 by two‐tailed unpaired t‐test with Welch's correction.
Source data are available online for this figure.
