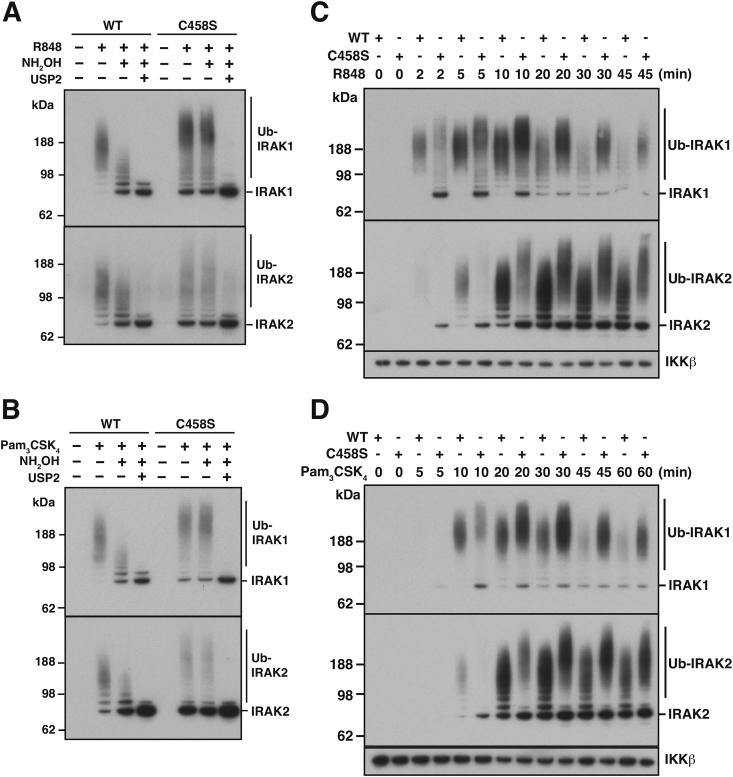
Fig. 4.
The ubiquitin chains attached to IRAK1 and IRAK2 during TLR signalling are initiated by the formation of ester as well as isopeptide bonds. (A, B) BMDM from WT or HOIL-1[C458S] knock-in mice were stimulated for 10 min with the TLR7 activating ligand 1 μg/ml R848 (A) or for 20 min with 1 μg/ml Pam3CSK4 an activator of the TLR1/2 heterodimer. The cells were lysed and ubiquitylated proteins captured from the cell extracts on Halo-NEMO beads and incubated for 30 min at 37 °C with λPPase. Following incubation for 60 min at pH 9.0 without (-) or with (+) 0.5 M hydroxylamine, the beads were incubated for a further 60 min at pH 7.5 without (-) or with (+) 1 μM USP2. Immunoblotting was performed with antibodies recognising IRAK1 or IRAK2. (C, D) Rate of formation of ubiquitylated IRAK1 and IRAK2 during TLR signalling. BMDM from WT or HOIL-1[C458S] mice were stimulated with 1 μg/ml R848 or 1 μg/ml Pam3CSK4 for the times indicated, lysed and ubiquitylated IRAK1 and IRAK2 captured from the cell extracts and treated with λPPase as in A, B. Phosphatase treatment was followed by immunoblotting with antibodies recognising IRAK1, IRAK2 and IKKβ (the latter acting as a loading control that binds to NEMO in a ubiquitin-independent manner). The figure is adapted from results presented in Kelsall et al. (2019).