Abstract
Pacemakers, implantable cardiac defibrillators, and cardiac resynchronization therapy devices are potentially life-saving treatments for a number of cardiac conditions, but are not without risk. Most concerning is the risk of a cardiac implantable electronic device (CIED) infection, which is associated with significant morbidity, increased hospitalizations, reduced survival, and increased healthcare costs. Recommended preventive strategies such as administration of intravenous antibiotics before implantation are well recognized. Uncertainties have remained about the role of various preventive, diagnostic, and treatment measures such as skin antiseptics, pocket antibiotic solutions, anti-bacterial envelopes, prolonged antibiotics post-implantation, and others. Guidance on whether to use novel device alternatives expected to be less prone to infections and novel oral anticoagulants is also limited, as are definitions on minimum quality requirements for centres and operators and volumes. Moreover, an international consensus document on management of CIED infections is lacking. The recognition of these issues, the dissemination of results from important randomized trials focusing on prevention of CIED infections, and observed divergences in managing device-related infections as found in an European Heart Rhythm Association worldwide survey, provided a strong incentive for a 2019 International State-of-the-art Consensus document on risk assessment, prevention, diagnosis, and treatment of CIED infections.
Keywords: Infection, Endocarditis, Microbiology, Cardiac implantable electronic devices, Implantable cardioverter-defibrillators, Pacemakers, Cardiac resynchronization therapy, Leads, Extraction, Re-implantation, EHRA consensus document
Table of contents
Introduction 516a
Scope of the consensus document 516a
Methodology 516a
Background and epidemiology 516a
Pathogenesis and microbiology of cardiac implantable electronic device infections 516b
Risk factors for cardiac implantable electronic device infection 516c
Risk stratification 516e
Prevention 516e
Pre-procedural measures 516e
Patient selection 516e
Lead management 516e
Patient factors 516e
Anticoagulation and antiplatelet drugs 516e
Appropriate environment 516e
Staff training 516e
Nasal swabs/S. aureus decolonization of patients 516e
Pre-procedure skin preparation 516h
Pre-procedure antibiotic therapy 516h
Peri-procedural measures 516h
Patient surgical preparation 516h
Good surgical technique 516h
Antibiotic envelope 516h
Local instillation of antibiotics or antiseptics 516h
Capsulectomy 516h
Closure 516h
Post-procedural measures 516i
Post-procedure antibiotic therapy 516i
Wound care 516i
Re-intervention 516i
Diagnosis of cardiac implantable electronic device infections and related complications 516i
Clinical findings 516i
Identification of the causative microorganisms 516i
Imaging 516k
Echocardiography 516k
Radiolabelled leucocyte scintigraphy, positron emission tomography, and computerized tomography 516l
Management of cardiac implantable electronic device infections: when, how, and where 516n
Cardiac implantable electronic device removal 516n
Antimicrobial therapy including long-term suppressive therapy 516p
Preventive strategies after cardiac implantable electronic device implantations, new re-implantations, and alternative novel devices 516r
Preventive strategies after cardiac implantable electronic device implantations 516r
Re-implantations 516r
Alternative novel devices 516s
Prognosis, outcomes, and complications of cardiac implantable electronic device infections 516s
Special considerations to prevent device-related infections (elderly, paediatrics, adult with congenital heart disease) 516t
Minimum quality requirements concerning centres and operator experience and volume 516u
Health economics for cardiac implantable electronic devices infections and strategies to reduce costs 516v
Divergent recommendations from different societies 516v
General definitions and minimal requirements of variables in scientific studies and registries 516v
Gaps of evidence 516aa
Summary of emerging messages and call for scientific evidence 516aa
References 516ac
Introduction
Scope of the consensus document
Pacemakers (PM), implantable cardiac defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices are life-saving treatments for a number of cardiac conditions. Device-related infection is, however one of the most serious complications of cardiac implantable electronic device (CIED) therapy associated with significant morbidity, mortality, and financial healthcare burden. Although many preventive strategies such as administration of intravenous (i.v.) antibiotic therapy before implantation are well recognized, uncertainties still exist about other regimens. Questions still remain such as the use of CIED alternatives expected to be less prone to infections and how to manage medication, such as anticoagulants during CIED surgery, and the role of minimum quality and volume requirements for centres and operators. The recognition of these gaps in knowledge, reports of new important randomized trials, observed divergences in managing device-related infections,1 and the lack of international consensus documents specifically focusing on CIED infections provided a strong incentive for a 2019 State-of-the-art Consensus document on risk assessment, prevention, diagnosis, and management of CIED infections. The aim of this document is to describe the current knowledge on the risks for device-related infections and to assist healthcare professionals in their clinical decision making regarding its prevention, diagnosis, and management by providing the latest update of the most effective strategies.
Methodology
This consensus document is an international collaboration among seven professional societies/associations, including the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), the European Association for Cardio-Thoracic Surgery (EACTS), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the International Society for Cardiovascular Infectious Diseases (ISCVID). The writing group consisting of 16 Task Force Members, were selected based on their expertise and medical specialty (12 cardiologists with varying subspecialties, 2 infectious disease specialists, 1 imaging specialist, and 1 thoracic surgeon), from 11 countries in 4 continents.
All experts undertook a detailed comprehensive literature search until May 2019 (human research published in English and indexed in major databases such as MEDLINE, EMBASE, the Cochrane Library, and others as required) related to studied patient cohort and CIED infection topics using relevant search terms related to the field and prior guidelines. Systematic reviews of published evidence for management of given conditions and clinical problems were performed. Members were asked to weigh the strength of evidence for or against a particular diagnostic instrument, procedure, or treatment, include estimates of expected health outcomes and assess risk–benefit ratios where data existed. Patient-, device-, and procedure-specific modifiers were considered, as were the results of the international survey on CIED infections conducted for this purpose1 and of previous registries.2 Consensus statements were evidence-based, derived primarily from published data and by consensus opinion after thorough deliberations, requiring at least 80% predefined consensus delivered via email by chairs to all expert members for their approval/rejection.
The EHRA user-friendly ranking system, for consensus documents, with ‘coloured hearts’ providing the current status of the evidence and consequent guidance was used for the coding of the scientific evidence for statements made (Table 1). The grading does not have separate levels of evidence, which instead are defined in each of the coloured heart grades. A letter coding ‘ROME’ defining existing scientific evidence was applied: R for randomized trials, O for observational studies, M for meta-analyses, and E for expert opinion (Table 1).
Table 1.
Scientific rationale of recommendations
| Consensus statement related to a treatment or procedure | Definitions of consensus statement | Statement class | Scientific evidence coding (SEC) | Ref. |
|---|---|---|---|---|
| Recommended/indicated or ‘should do this’ | Scientific evidence that a treatment or procedure is beneficial and effective. Requires at least one randomized trial, or is supported by large observational studies and authors’ consensus |

|
R | |
| May be used or recommended | General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. May be supported by randomized trials based on small number of patients or not widely applicable |

|
O | |
| Should NOT be used or recommended | Scientific evidence or general agreement not to use or recommend a treatment or procedure |

|
E |
This categorization for the consensus document should not be considered as being directly similar to that used for official society guideline recommendations which apply a classification (I–III) and level of evidence (A, B, and C) to recommendations.
The ‘ROME’ coding was applied for each consensus statement, defining existing scientific evidence.
E, expert opinion; M, meta-analyses; O, observational studies; R, randomized trials.
The document was peer-reviewed by official external reviewers representing EHRA, the participating societies, and ESC Committee for Practice Guidelines (CPG). All members of the writing group as well as reviewers have disclosed potential conflicts of interest, at the end of this document.
Since this consensus document includes evidence and expert opinions from various countries and healthcare systems, the medical approaches discussed may include drugs or devices that are not approved by governmental regulatory agencies in all countries. Moreover, the ultimate decision on management must be made by the healthcare provider and the patient in light of individual factors presented.
Background and epidemiology
Over the last decades, there has been a substantial increase in the number and complexity of CIED implantations as a result of expanded indications and progressive aging of the population. Although these devices improve cardiovascular outcomes, they also expose patients to a risk for potential complications.
Infection is one of the most serious complications of CIED therapy and is associated with significant mortality, morbidity, and financial healthcare burden. It is difficult to give a precise rate of CIED infections because of divergent definitions, varied populations, and the range of rates in retrospective and prospective studies. In the Danish registry including 46 299 consecutive patients who underwent pacemaker implantation between 1982 and 2007, the incidence of infection was 4.82/1000 device-years after a primary implantation, and 12.12/1000 device-years after replacement.3 Greenspon et al. found that the incidence of CIED infection in the USA increased from 1.53% in 2004 to 2.41% in 20084 and a National Inpatient Sample database study showed an increase from 1.45% to 3.41% (P < 0.001) from 2000 through 2012, particularly for CRT devices.5 Infection rates in prospective observational studies,6,7 registries8 and more recent cross-over cluster PADIT- and randomized WRAP-IT trials,9,10 were only 0.6–1.3%, as compared to retrospective studies,11,12 reporting significantly higher rates (2.3–3.4%) in the first year after implantation.
Pathogenesis and microbiology of cardiac implantable electronic device infections
Cardiac implantable electronic device infections occur via two major mechanisms. The most common is contamination of leads and/or pulse generator during implantation or subsequent manipulation.13 Device erosion late after interventions may either be due to, or result in pocket infection. In either case, contamination and subsequent bacterial colonization result in pocket infection which can spread along the intravascular parts of the leads and progress to systemic infection. The second mechanism is a bloodstream infection.14 Direct lead seeding can occur during bacteraemia caused by a distant infectious focus, such as a local septic thrombophlebitis, osteomyelitis, pneumonia, surgical site infection, contaminated vascular catheters or bacterial entry via the skin, mouth, gastrointestinal, or urinary tract.
Factors, which play a role in the pathogenesis of CIED infections, can be related to the host, the device, or the microorganism. The patient’s own skin flora can be introduced into the wound at the time of skin incision and thereby contaminate the device. Contamination may also occur before implantation via the air in the operating room (both host and staff) or via the hands of anyone handling the device. From a pathophysiological standpoint, device-related factors are those affecting bacterial adherence to the generator or lead and the biofilm formation on these surfaces. Bacterial adherence is facilitated by irregular and hydrophobic surfaces.15 Of the commonly used polymers, polyvinylchloride and silicone allow better adherence than polytetrafluoroethylene, while polyurethane allows less adherence than polyethylene. Metals also differ in their propensity for bacterial adherence—e.g. titanium has less propensity for bacterial adherence than steel. Normally non-pathogenic microorganisms such as Coagulase-negative Staphylococci (CoNS) may adhere to the CIED and establish a focus of infection. The microorganisms most frequently isolated have been Gram-positive bacteria (70–90%), especially CoNS (37.6% of the isolates) and Staphylococcus (S.) aureus (30.8%), which are far more prone to adhere to non-biological material than others (Table 2).16,17,19Staphylococcus aureus is the most common cause of bacteraemia and early pocket infections. Altogether, methicillin-resistant staphylococci were isolated in 33.8% of CIED infections (49.4% of all staphylococcal infections),16 their frequency varied by country, and even hospital.18,20 Over the past decade the rates of methicillin resistance seem to be greater than those reported earlier.16 Gram-negative bacteria were isolated in 8.9% while other microbes such as streptococci, anaerobes, and fungi were less often isolated (Table 2). Enterobacteriaceae, other Gram-negative rods and fungi were rare (Table 2).
Table 2.
Pathogens isolated in patients undergoing interventions for device infection from three large patient cohorts in North America, Europe, and Asia
| Percentage of isolates |
|||
|---|---|---|---|
| Pathogen | North America16 | Europe17 | Asia18 |
| Coagulase-negative staphylococci | 69 | 45.2 | |
| Methicillin-resistant | 18.8 | ||
| Methicillin-sensitive | 18.8 | ||
| S. aureus | 13.8 | 4.1 | |
| Methicillin-sensitive | 15.8 | ||
| Methicillin-resistant | 15.0 | ||
| Streptococcus spp. | 2.5 | ||
| Enterococcus spp. | |||
| Vancomycin-sensitive | 2.8 | ||
| Vancomycin-resistant | 1.4 | ||
| Cutibacterium spp. (previously Propionibacterium spp.) | 2.5 | ||
| Corynebacterium | 5 | ||
| Gram-negative bacteria | 8.9 | 6.1 | 9.1 |
| Enterobacteriaceae | 3 | 3.2 | |
| Non-fermentative bacilli, incl. Pseudomonas spp. | 1.5 | 5.9 | |
| Anaerobes | 1.6 | ||
| Fungi | 0.9 | 1 | 0.9 |
| Mycobacteria | 0.2 | ||
Risk factors for cardiac implantable electronic device infection
Risk factors for CIED infection may be divided into patient-related, procedure-related, and device-related factors. These risk factors may be modifiable or non-modifiable. Identification of modifiable risk factors is important because they may allow for preventive measures to reduce the risk. In patients with non-modifiable risks, alternative approaches may be an option to lower the overall risk. For example, renal dialysis is a non-modifiable patient risk factor. By changing the procedure and/or device and selecting an epicardial or subcutaneous system the risk may be reduced. Several studies have examined large databases for the most common risk factors. A meta-analysis21 of pooled data including 206 176 patients in 60 studies (of which 21 were prospective and 39 retrospective) is presented in Table 3. Other large studies analysing risk factors include device registry data matched with Medicare fee-for-service claims data,22 the National Inpatient Sample database study with 85 203 device-related infections,5 and the recent Danish device-cohort study, including 97 750 patients.23
Table 3.
Pooled effect estimates for potential risk factors predisposing to cardiac implantable electronic device infection
| Prospective + retrospective studies |
Prospective studies only |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Studies (n) | Total (n) | Pooled estimate | P-value | Studies (n) | Total (n) | Pooled estimate | P-value |
| Patient-related factors | ||||||||
| ESRDa | 8 | 3045 | 8.73 [3.42, 22.31] | 0.00001 | NA | |||
| History of device infection | 4 | 463 | 7.84 [1.94, 31.60] | 0.004 | NA | |||
| Fever prior to implantation | 3 | 6652 | 4.27 [1.13, 16.12] | 0.03 | 2 | 6580 | 5.34 [1.002, 28.43] | 0.05 |
| Corticosteroid use | 10 | 3432 | 3.44 [1.62, 7.32] | 0.001 | 3 | 1349 | 2.10 [0.47, 9.32] | 0.33 |
| Renal insufficiencyb | 5 | 2033 | 3.02 [1.38, 6.64] | 0.006 | NA | |||
| COPD | 6 | 2810 | 2.95 [1.78, 4.90] | 0.00003 | 2 | 2393 | 2.30 [0.97, 5.48] | 0.06 |
| NYHA class ≥ 2 | 3 | 2447 | 2.47 [1.24, 4.91] | 0.01 | 2 | 2393 | 2.77 [1.26, 6.05] | 0.01 |
| Skin disorders | 4 | 6810 | 2.46 [1.04, 5.80] | 0.04 | 2 | 6519 | 2.60 [0.88, 7.70] | 0.08 |
| Malignancy | 6 | 1555 | 2.23 [1.26, 3.95] | 0.006 | NA | |||
| Diabetes mellitus | 18 | 11839 | 2.08 [1.62, 2.67] | <0.000001 | 7 | 9815 | 1.88 [1.19, 2.98] | 0.007 |
| Heparin bridging | 2 | 6373 | 1.87 [1.03, 3.41] | 0.04 | NA | |||
| CHF | 6 | 1277 | 1.65 [1.14, 2.39] | 0.008 | NA | |||
| Oral anticoagulants | 9 | 8527 | 1.59 [1.01, 2.48] | 0.04 | 3 | 7271 | 1.18 [0.44, 3.11] | 0.75 |
| Procedure-related factors | ||||||||
| Procedure duration | 9 | 4850 | 9.89 [0.52, 19.25] | 0.04 | 6 | 4508 | 13.04 [-0.64, 26.73] | 0.06 |
| Haematoma | 12 | 14228 | 8.46 [4.01, 17.86] | <0.000001 | 6 | 9715 | 9.33 [2.84, 30.69] | 0.0002 |
| Lead repositioning | 5 | 1755 | 6.37 [2.93, 13.82] | 0.000003 | 4 | 1659 | 7.03 [2.49, 19.85] | 0.0002 |
| Inexperienced operatorc | 2 | 1715 | 2.85 [1.23, 6.58] | 0.01 | 2 | 1715 | 2.85 [1.23, 6.58] | 0.01 |
| Temporary pacing | 10 | 10683 | 2.31 [1.36, 3.92] | 0.002 | 4 | 8683 | 3.29 [1.87, 5.80] | 0.00004 |
| Device replacement/revision/upgrade | 26 | 21214 | 1.98 [1.46, 2.70] | 0.00001 | 8 | 8793 | 0.95 [0.49, 1.87] | 0.89 |
| Generator change | 20 | 12134 | 1.74 [1.22, 2.49] | 0.002 | 6 | 2139 | 0.91 [0.37, 2.22] | 0.83 |
| Antibiotic prophylaxis | 16 | 14166 | 0.32 [0.18, 0.55]d | 0.00005 | 11 | 10864 | 0.29 [0.13, 0.63] | 0.002 |
| Device-related factors | ||||||||
| Epicardial leads | 3 | 623 | 8.09 [3.46, 18.92] | 0.000001 | NA | |||
| Abdominal pocket | 7 | 4017 | 4.01 [2.48, 6.49] | <0.000001 | 2 | 2268 | 5.03 [1.96, 12.91] | 0.0008 |
| ≥2 leads | 6 | 1146 | 2.02 [1.11, 3.69] | 0.02 | NA | |||
| Dual-chamber device | 14 | 45224 | 1.45 [1.02, 2.05] | 0.04 | 7 | 12102 | 1.28 [0.73, 2.25] | 0.38 |
Risk parameters which were statistically significant for retrospective and prospective data are shown. Analyses restricted to prospective data only for the same parameters (if available) are also shown. Adapted from Polyzos et al.21
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; NA, not available; NYHA, New York Heart Association.
GFR ≤15 mL/min or haemodialysis or peritoneal dialysis.
Glomerular filtration rate (GFR) <60 mL/min or creatinine clearance (CrCL) <60 mL/min.
<100 previous procedures.
The pooled effect estimate from randomized studies was 0.26 [0.13, 0.52].
A summary of the most important risk factors identified in these trials are listed in Table 3 (adapted from Polyzos et al.21) Unfortunately, the importance of risk factors varied from study to study and in some cases findings were contradictory (age as an example).
Of the patient-related factors, end-stage renal disease was consistently associated with the highest risk, underscoring the importance of a careful clinical evaluation in these patients. In the meta-analysis risk factors included: end-stage renal disease, renal insufficiency, diabetes mellitus, chronic obstructive pulmonary disease, corticosteroid use, history of previous device infection, malignancy, heart failure, pre-procedural fever, anticoagulant drug use, and skin disorders, but not age or gender.21 However younger age, along with prior device infection were identified as significant risks in the Danish device-cohort study.23 Others identified malnutrition (OR 2.44, P < 0.001) as a strong risk factor.5
Regarding procedure-related factors, antibiotic prophylaxis was associated with a 70% relative risk reduction in infection and is now the standard of care.21 The presence of a haematoma was associated with an approximately nine-fold increased risk of infection. These findings were later confirmed by the prospective BRUISE-CONTROL study, which reported data from 659 patients in whom there was a hazard ratio of infection of 7.7 (95% CI 2.9–20.5; P < 0.0001) in case of clinically significant haematoma (requiring surgery and/or resulting in prolonged hospitalization ≥24 h, and/or requiring interruption of anticoagulation), with as many as 11% of these patients developing this complication over 1-year follow-up.24 Early reoperation for haematoma or lead dislodgement were identified as the strongest risk factors for CIED infection in a device registry data matched with Medicare fee-for-service claims data.22 Haematoma was also one of the strongest risk factors (OR 2.66, P < 0.001) in a National Inpatient Sample database study.5 Procedure duration was associated with a multifold increased risk of infection, although there was significant heterogeneity in the studies.21 Data from the Danish device registry23 showed that compared to procedures lasting <30 min, the relative risk [95% CI] of infection for procedures lasting 60–90, 90–120, or >120 min were 1.54 [1.24–1.91], 1.85 [1.36–2.49], and 2.42 [1.77–3.33], respectively. The same registry identified implantation of CRT and reoperations as high and significant risks.23 Another study confirmed early lead repositioning as a strong predictor of infection although it is as yet unknown whether delaying the re-intervention would reduce risk.21 Temporary pacing has also been shown to increase the risk of infection21 (and carries a risk of perforation/tamponade). This may be due to deviations in sterility measures due to urgent placement, need for lead re-manipulation and simply as a chronic portal of entry to the bloodstream. Indication for temporary transvenous pacing should therefore be carefully considered, and alternative measures such as backup transthoracic pacing or infusion of rate-accelerating drugs evaluated. Device generator replacement roughly doubles the risk of infection, possibly due to activation of pre-existing bacterial colonization or reduced penetration of antibiotics into the encapsulated generator pocket.21 As with any procedure, experience has an impact on outcome,25 and risk of infection may be increased by allocating generator changes to inexperienced operators.
There are fewer device-related factors for CIED infection. After restricting analysis to prospective studies, an abdominal pocket was the only significant risk factor,21 although factors such as patient profile and type of intervention may have confounded the results. Data from the Danish registry23 showed that device complexity and the numbers of leads were factors significantly associated with increased infection risk on multivariate analysis with a HR of 1.26, 1.67, and 2.22 for ICD, CRT-P, and CRT-D systems, respectively as compared to PMs (P ≤ 0.002 for all comparisons).
Risk stratification
Considering that CIED infections occur in the presence of multiple host and procedure-related factors, risk scores have been developed to identify patients at low and high risk. Scoring systems could play a role in better identifying patients at risk than individual factors, especially considering the inconsistency of the reported factors in various studies.
A single centre study of 2891 ICD or CRT-D recipients identified a novel composite score of 7 independent risk factors for infection and defined patients as low (1% risk), medium (3.4%), and high (11.1%) risk for infection.26 Related to its moderate predictive range, the model has not been adopted for risk stratification. Another study identified 10 preoperative risk factors associated with CIED infection for a risk score system that defined score <1 as low risk (1%) and ≥3 as high risk (infection rate 2.4%).27 Despite the potential practical use of such risk scores, they can currently not be recommended because the evidence behind them remains weak.
Prevention
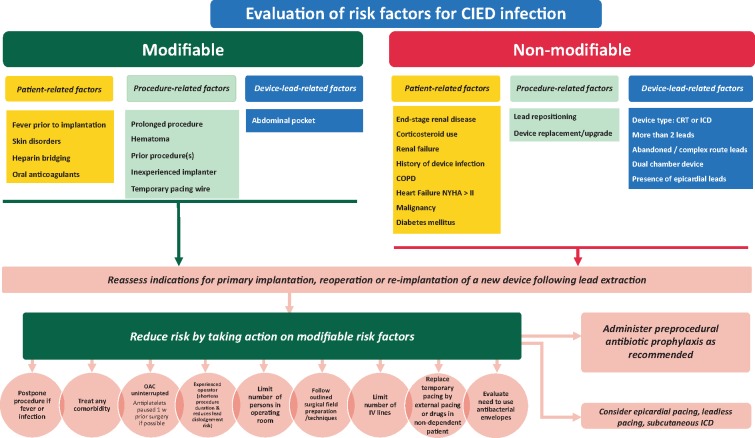
A summary of recommended preventive measures is shown in Table 4. A flowchart that indicates how modifiable risk factors can be minimized on various levels is shown in Figure 1.
Table 4.
List of recommended preventive measures for CIED infections
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| Pre-procedural measures | |||
| Confirm indication for CIED |

|
E | |
| Delay CIED implantation in patients with infection |

|
E | 28 |
| Avoid temporary transvenous pacing and central venous lines, which should ideally be removed prior to introducing new hardware, whenever possible |

|
O, M | 21 |
| Measures to avoid pocket haematoma are recommended (avoid heparin bridging, discontinue antiplatelets if possible) |

|
R | 21 , 29–31 |
| Periprocedural use of therapeutic low-molecular-weight-heparin |

|
R, M, O | 30 , 32 , 33 |
| Perform the CIED procedure in an operating room/suite with complete sterile environment as required for other surgical implant procedures |

|
E | 34 |
| Procedure should be performed or supervised by an operator with sufficient training and experience (Table 12) |

|
O | 45 |
| Topical S. aureus decolonization may be performed |

|
E | |
| Pre-procedural skin wash may be performed |

|
E | |
| Hair removal with electric clippers (not razors) is recommended |

|
O | 35 |
| Antibiotic prophylaxis is recommended within 1 h of incision for cefazolin and flucloxacilline, within 90-120 min for vancomycin |

|
R, M | 21 |
| A continuous surveillance program of infection rates and associated microbiology should be set-up at the level of each implanting centre |

|
E | – |
| Peri-procedural measures | |||
| Surgical preparation with alcoholic chlorhexidine should be used rather than povidone-iodine |

|
R | 36 , 37 |
| Allow sufficient time for the antiseptic preparation to dry |

|
E | |
| Adhesive iodophor-impregnated incise drapes may be used |

|
E | |
| Perform the procedure with adequate surgical technique—minimize tissue damage, haemostasis, adequate wound closure |

|
E | |
| Antibiotic envelope in high-risk situations is recommendeda |

|
R | 10 |
| If the operator performs the prepping and draping, glove change/re-scrub or remove outer glove of a double-glove before incision |

|
E | |
| Using local instillation of antiseptic and antibiotics in the pocket |

|
R, E | 9 |
| Use of braided sutures for final skin closure |

|
E | |
| Post-procedural measures | |||
| Use of postoperative antibiotic therapy |

|
R | 9 |
| Adequate dressing for 2–10 days is recommended |

|
E | |
| Patient instructions on wound care should be provided |

|
E | |
| Delay or reconsider indication for re-intervention if possible |

|
E | |
| Haematoma drainage or evacuation (unless tense, wound dehiscence is present or pain is severe) |

|
O | 24 , 28 |
Candidates are those as defined in the WRAP-IT study population10 (patients undergoing pocket or lead revision, generator replacement, system upgrade, or an initial CRT-D implantation) and patients with other high risk factors as outlined in Table 3, considering also the local incidence of CIED infections.
CIED, cardiac implantable electronic device; E, expert opinion; M, meta-analysis; O, observational studies; R, randomized trials.
Figure 1.
A flowchart indicating how device-related infections can be minimized by targeting modifiable risk factors on various levels. Risk factors ranked in order of strength from top to bottom. CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; NYHA, New York Heart Association; OAC, oral anticoagulation; w, week.
Pre-procedural measures
Patient selection
The best treatment of device-related infections is prevention. Careful consideration should be given to whether the risks of device implantation, in any individual patient, outweighs the benefit. If there is a significant risk of infection delay of implantation for a period of observation or longer-term antibiotic treatment might be of value. For patients undergoing device removal for infection, one-third to one-half may not require device re-implantation.38 If the decision is to proceed with an implantation, it is important to ‘think before you choose’. Avoiding a transvenous system, and implanting an epicardial system, may be preferential in high-risk patients.39 There is hope that ‘leadless’ pacemakers will be less prone to infection and can be used in a similar manner in high-risk patients.40,41 Subcutaneous ICDs (S-ICD) are an option in patients requiring sudden death protection without requiring pacing. Decisions must be made on an individual basis, weighing all known risks and benefits.
Lead management
The number of leads and the presence of abandoned leads are associated with increased risk for complications, including infection. The decision to abandon or extract a lead can be complex and must be made on an individual basis weighing all known risks and benefits. The increased risk of infection, and increased risk of extraction if an infection occurs, must be considered in this decision.42,43
Patient factors
In patients who have fever or signs of active infection, a procedure should be delayed until a patient has been afebrile for at least 24 h.28 The need for temporary pacing wires increases the risk of infection and should be avoided if possible.28 Temporary pacing via a jugular route may provide a lower risk of infection than groin access, although this remains to be proven. Studies have demonstrated that better glycaemic control in the peri-procedural period may reduce infections in surgical patients.44
Anticoagulation and antiplatelet drugs
The development of a pocket haematoma increases the risk for infection.24 Studies have demonstrated that a ‘bridging’ approach with anticoagulation increases the risk of haematoma and is no longer recommended.30 In patients who are not at high risk for thrombo-embolic events (e.g. CHA2DS2VASc score <4), holding anticoagulation for the procedure and restarting when the bleeding risk is reduced seems prudent. In higher-risk patients, such as those with prior embolic event or mechanical valve, continuing anticoagulation with Warfarin is recommended. Preliminary data from the BRUISE-Control 2 study suggests the same may be true for non-vitamin K antagonist oral anticoagulants.29 Therapeutic low-molecular-weight-heparin (LMWH) should be avoided.30,32,33 Antiplatelet agents, especially P2Y12 inhibitors (clopidogrel, prasugrel, ticagrelor) significantly increase the risk for bleeding and should (unless clearly indicated) preferably be discontinued for 5–10 days before the intervention, especially if they are combined with oral anticoagulation.31
Appropriate environment
Both in operating rooms and Electrophysiology/Catheterization laboratories, the standards for sterile procedures (e.g. cleaning, room design, ventilation, limitation of area traffic, etc.) must be met as for other surgical procedures associated with implants. Minimum standards for the environment for CIED procedures have been published.41 It is recommended that each centre set up a continuous surveillance program of their infection rates and flora involved. Data must be correlated with patient, procedure, staff, and device information (Table 4).
Staff training
All staff involved in CIED implantation must be trained in appropriate strict sterile techniques and behaviour in an operating room setting (scrubbing, set up of tables, patient preparation, and strict limitation to room traffic). Operators should be adequately trained45 and supervised.
Nasal swabs/S. aureus decolonization of patients
For elective procedures, S. aureus colonization can be detected by nasal swabs. Nasal treatment with mupirocin and chlorhexidine skin washing can reduce colonization and has been shown in some surgical studies to reduce the risk for infection,46 but there are no studies relating specifically to CIED interventions.
Pre-procedure skin preparation
In many hospitals, pre-surgical washing with an anti-microbial agent is employed. The data on this practice for general surgical procedures are diverse and a recommendation for its routine use therefore can not be strongly supported.47 If chest hair needs to be removed, electric clippers with a single-use head (and not razors) should be used on the day of the procedure.35
Pre-procedure antibiotic therapy
The use of prophylactic systemic antibiotics has been proven to lower infection rates of CIED and is the standard of care.48,49 It significantly reduces the incidence of device infection, compared with no antibiotic therapy, with a 40–95% relative risk reduction.21 Antibiotics must be completed within 1 h of incision to ensure adequate tissue levels. Staphylococcus aureus is the most common organism involved in acute CIED infections. The degree of methicillin resistance varies. Antibiotics should at least cover S. aureus species. Currently, there are no significant data to support routine Methicillin-Resistant S. Aureus (MRSA) coverage and its usage should be guided by the prevalence of MRSA in the implanting institution and patient risk. Randomized trials have used i.v. flucloxacillin (1–2 g) and first-generation cephalosporins such as cefazolin (1–2 g).9,48,49 Vancomycin (15 mg/kg) may be used in case of allergy to cephalosporins and since it should be administered slowly (approximately over 1 h) it needs to be started 90-120 min prior to the incision.
Peri-procedural measures
Patient surgical preparation
Randomized studies have demonstrated alcoholic 2% chlorhexidine to be superior to povidone-iodine (with or without alcohol) for skin preparation prior to surgery36 or intra-vascular catheter insertion37 but no randomized data exist regarding CIED implantation. The antiseptic should be allowed to dry completely before incision, in order to provide sufficient time for it to be effective. In addition, alcoholic antiseptic agents may carry a fire hazard with electrocautery, especially if there is pooling. Many operators use adhesive incise drapes, but there is no evidence that it reduces infection rates (and may even increase risk of infection when non-iodophor incise drapes are used50).
Good surgical technique
Minimizing tissue damage, strict attention to haemostasis, and adequate wound closure are all important measures to reduce infection. Many operators change gloves (e.g. by double-gloving) when draping the patient and also before handling the generator. Non-powdered gloves may reduce the risk of infection by reducing local inflammation.51 Pocket haematoma is associated with an increased risk of infection.24 There are no data supporting the routine use of topical haemostatic agents, although, they may be useful in selected patients. Vigorous pocket irrigation is important to remove devitalized tissue as well as dilute any contaminants.52 Diagnostic or therapeutic aspiration of a haematoma is contraindicated given the risk of ‘inoculating’ the pocket and causing an infection.24,28 Haematoma evacuation should only be undertaken if pain is unmanageable or wound closure is threatened, and should ideally be performed in an operating room.24
Antibiotic envelope
An antibacterial mesh envelope [TYRX™, Medtronic, MN, USA] has been developed, which locally releases minocycline and rifampin for a minimum of 7 days to prevent infections and biofilm formation and is fully absorbed in ∼9 weeks. The WRAP-IT trial10 has shown that the envelope significantly reduces the incidence of CIED infection in high-risk patients (undergoing pocket or lead revision, generator replacement, system upgrade, or an initial CRT-D implantation) without a higher incidence of complications. A total of 6983 patients were randomized to receive the envelope or not, with a lower incidence of primary endpoints (infection resulting in system extraction or revision, long-term antibiotic therapy, or death) within 12 months after the CIED implantation in patients who received the envelope vs. controls: 0.7% and 1.2%, respectively (hazard ratio 0.60; 95% confidence interval 0.36–0.98; P = 0.04).10 While the population treated showed benefit, the number of patients needed to treat to prevent one infection was high. The exclusion of higher-risk patients (those treated with immunosuppressive treatments, with vascular access, or on dialysis) may have contributed to a lower-than-expected rate of infections (1.2%) also observed in other prospective studies.6,7,9 A heightened awareness of infection prevention when participating in prospective trials may also explain such low rates. Higher infection rates (2.3–3.4%), as observed in less-selected retrospective studies,11,12 would improve the overall cost-effectiveness of the envelope. Recommendation for the use of the antibacterial envelope is outlined in Table 4. The use should be individualized based upon presence of risk factors (Table 3) and the local incidence of CIED infections.
The use of other ‘envelopes’ (bioscaffold or pericardium patches) for stabilization, antibiotic soaked gauze, etc. has not been rigorously studied and cannot be supported.
Local instillation of antibiotics or antiseptics
While vigorous pocket irrigation is recommended the use of local installation of an antibiotic or antiseptic is not. The recent PADIT trial demonstrated no benefit (see below).9
Capsulectomy
Even in the absence of signs of clinical infections, cultures taken at the time of generator change demonstrate a significant incidence of colonization.53 In addition, the fibrous capsule inhibits the body’s normal defence mechanisms and antibiotic penetration. Theoretically, ‘capsulectomy’ mitigates these issues but could also result in more pocket bleeding/haematoma, and therefore cannot be recommended as routine practice.54
Closure
Wound dehiscence or superficial infection can lead to a frank pocket infection. Closure in layers minimize wound tension and reduces the risk of dehiscence and infection.55 Skin closure can be with a subcuticular absorbable suture, non-absorbable suture, surgical staples, or surgical adhesive. If non-absorbable material is used, it must be removed in a timely manner when clinically appropriate (usually 7–14 days). Absorbable sutures must be placed with care to allow for absorption and avoidance of a ‘stitch abscess’ especially at the site of the knot. Although there are no data indicating that the type of suture material impacts the risk of infection, many operators prefer non-braided monofilament sutures for skin closure as they may avoid bacterial adhesion (see Pathogenesis and microbiology of cardiac implantable electronic device infections section). Some sutures are impregnated with antibiotics, but since there is no evidence that it reduces infection, it cannot be recommended over standard sutures.
Post-procedural measures
Post-procedure antibiotic therapy
Some physicians administer post-implant antibiotics from a single dose to a week i.v. and oral administration.1 The recent PADIT trial,9 with its cluster cross-over design, tested the clinical effectiveness of incremental perioperative antibiotics to reduce device infection. The conventional treatment was a single-dose preoperative cefazolin infusion vs. a combination of pre-procedural cefazolin plus vancomycin, intra-procedural bacitracin pocket wash, and 2-day postoperative oral cephalexin in almost 20 000 patients undergoing CIED implantation. The primary outcome of 1-year hospitalization for device infection in the high-risk group was not statistically significant (non-significant 20% reduction of infection). The device infection rates were low. As there are no data supporting this practice, it is not recommended to administer postoperative antibiotic therapy.
Wound care
An appropriate dressing should cover the incision at the end of the operation (except in the case of surgical adhesive). Clinical practice varies with the dressing being left on for 2–10 days. Pressure dressing may be used for the first 24 h to avoid haematoma. It is not necessary to change the dressing, unless it becomes impregnated. Some dressings are waterproof and allow the patient to shower. Patients should be advised to avoid soaking the wound (e.g. by swimming) until it is entirely healed (which usually takes approximately a month). They should also be instructed to seek medical attention in case of signs of local infection.
Re-intervention
It is well known that early re-intervention dramatically increases the risk of infection,19,21,28 so all measures must be taken to avoid this need (i.e. avoid haematoma, lead dislodgment, etc.). Some operators delay re-intervention by weeks (e.g. for lead repositioning) in an attempt to reduce this risk. This strategy may also alleviate the pain associated with early re-intervention, but further research is needed to determine whether this effectively reduces the risk of infection.
Diagnosis of cardiac implantable electronic device infections and related complications
Clinical findings
A superficial incisional infection should be differentiated from a pocket infection, as it involves only the skin and the subcutaneous tissue without communication with the pocket (and hence does not require CIED system extraction).56,57 Close monitoring of the patient must be pursued in order to recognize early recurrence that may be a sign of a significant pocket infection.
Pocket infection is defined as an infection limited to the generator pocket. It is clinically associated with local signs of inflammation that may be mild and characterized by erythema, warmth, and fluctuation.14,57 Deformation of the pocket, adherence or threatened erosion are often signs of low grade, indolent infection. Symptoms and signs of an infected surgical wound may fluctuate and although it can be difficult to recognize initially it is not recommended to take a sample of pocket material. Once a wound dehiscence occurs, a purulent drainage or a sinus is established, and a pocket infection is clearly present. If the generator or proximal leads are exposed, the device should be considered infected, irrespective of the results of the microbiology. Material from the pocket may be used for culture, recognizing the potential for contamination. Pocket infections may be associated with lead infections and CIED systemic infections and/or infective endocarditis. The actual rates depend on the definitions used in different studies.58
The diagnosis of CIED systemic infection and infective endocarditis without local infection may be more challenging (Table 5). Symptoms may be non-specific (fever, chills, night sweats) and a long period of time may elapse between CIED implantation and symptom onset as well as diagnosis. Patients with CIED infection may present with embolic involvement of lungs and pleural space, frequently misdiagnosed as pulmonary infections.61,62 Cardiac implantable electronic device infections may also be revealed by other distant foci as vertebral osteomyelitis and discitis. C-reactive protein (CRP) may be helpful although non-specific and procalcitonin (PCT) test may be of value, especially if positive (≥0.05) due to the high specificity for pocket infection compared to no infection and in case of embolic phenomena and S. aureus endocarditis.63,64
Table 5.
Recommendations for diagnosis of CIED infections and/or infective endocarditis: the Novel 2019 International CIED Infection Criteria
| Consensus statement | Statement class | Scientific evidence coding | Reference | |
|---|---|---|---|---|
| ||||
| Major criteria |

|
E | 59 | |
| Microbiology |
|
|||
| ||||
| ||||
| Imaging positive for CIED infections and/or IE |
|
|||
| ||||
| ||||
| Minor criteria |

|
E | 59 | |
| ||||
| ||||
| ||||
| ||||
Based on merging of the modified Duke and ESC 2015 Guidelines criteria, see text.59,60 Green text refers to CIED-related infection criteria.
CIED, cardiac implantable electronic device; CT, computerized tomography; E, expert opinion; ICE, intracardiac echocardiography; IE, infective endocarditis; M, meta-analysis; O, observational studies; R, randomized trials; SPECT, single-photon emission tomography; WBC, white blood cell.
There is no standardized diagnostic tool for CIED endocarditis. At present, the modified Duke criteria60 and the ESC 2015 criteria59 for the diagnosis of infective endocarditis are the only available framework for CIED endocarditis diagnosis. However, none represent a validated and standardized tool for diagnosis in this specific setting. In order to increase sensitivity for CIED infection diagnosis, this panel recommends additional criteria and to merge the modified Duke criteria60 and the ESC 201559 criteria. As a result, the 2019 International CIED Infection Criteria have been developed, and are detailed in Table 5.
Identification of the causative microorganisms
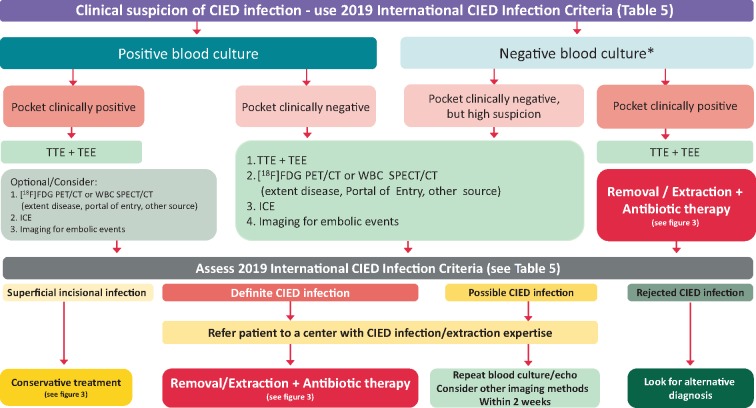
Identification of the causative microorganisms for a CIED infection is pivotal for effective antibiotic therapy (Table 2). Therefore, every effort should be made to obtain cultures prior to the institution of antibiotic therapy. Blood cultures should be repeated in patients with CIED and fever without clear signs of local infections and infective endocarditis. Three sets of blood cultures should be taken (at least 30 min in between) prior to starting antibiotic therapy (Table 6). Multiple blood cultures at different time intervals enable a distinction between transient and persistent bacteraemia and increases sensitivity. In stable patients, a 2–3 days washout period free from antibiotic therapy may increase precision of microbiological diagnosis. In unstable patients with sepsis or septic shock, early empiric antibiotic therapy should be administered following two sets of blood cultures thus not delaying start of antibiotic therapy. Blood bottles must be filled properly in order to increase the sensitivity.17,65 An aseptic technique for blood culture is mandatory since bacteria mostly considered as skin contaminants often are the causative agents of CIED infections. Every positive blood culture, including a single bottle with CoNS or other Gram-positive organisms, should be carefully evaluated and prompt active exclusion of CIED infection with other diagnostic techniques employed (Figure 2).71 In case of negative blood cultures (usually 5 days), increased incubation time (10–14 days) and the use of biomolecular methods (DNA amplification and/or gene sequencing) to detect fastidious or atypical pathogenes19 may be considered for CIED endocarditis and persistent negative blood cultures (Table 6).67 Among Gram-positive microorganisms there are species that may require longer period of incubation, such as Cutibacterium (previously Propionibacterium) acnes, especially in anaerobic condition.19 It has been postulated that S. aureus may be associated with earlier infections and with infective endocarditis compared to other pathogens, but data are still inconsistent. More severe cases may be due to S. aureus and Gram-negative rods.
Table 6.
Recommendations for diagnosis of CIED infections by clinical findings and microbiology
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| At least three sets of blood cultures should be acquired in case of clinically suspected CIED endocarditis |

|
E, O | 19 , 65 |
| Samples from the pocket should be cultured but only if acquired during removal and not passing through the sinus |

|
E, O | 19 , 65 |
| Suspect CIED infections in case of vertebral osteomyelitis and/or embolic pneumonia (clinical signs and symptoms of CIED systemic infections may be difficult to recognize as only fever may be present) |

|
E, O | 61 , 65 |
| Cultures of extracted CIED should be performed |

|
E, O | 66 |
| PCT may be useful in case of infective endocarditis and embolism and/or in case of S. aureus CIED-related infective endocarditis |

|
E, O | 64 |
| Increased incubation time (10–14 days) for slowly-growing microorganism may be considered in case of CIED-related infective endocarditis and persistent negative blood cultures |

|
E | 67 |
| The usefulness of sonication of CIED to enhance microbial detection during removal/extraction is still under evaluation but may be used with caution when interpreting results |

|
E, O | 68–70 |
| Cultures from the sinus of the CIED pocket or from parts of the device exposed |

|
E | 19 |
CIED, cardiac implantable electronic device; E, expert opinion; M, meta-analysis; O, observational studies; PCT, procalcitonin; R, randomized trials.
Figure 2.
Diagnostic algorithm for diagnosis of suspected CIED infections. *, ensure sufficient number of blood cultures collected and absence of confounding antibiotic therapy prior to cultures. CIED, cardiac implantable electronic device; [18F]FDG PET/CT, fluorodeoxyglucose positron emission tomography—computed tomography; ICE, Intracardiac echocardiography; IE, infective endocarditis, TEE, transoesophageal echocardiography; TTE, transthoracic echocardiography; WBC SPECT/CT, white blood cell single-photon emission computed tomography—computed tomography.
Swabs collected from the chronic draining sinus or fistula for culture are discouraged (Table 6). Instead, tissue or fluid collected from the pocket via an adjacent intact portion of the skin (via a sterile needle or syringe) is encouraged avoiding passing through the sinus. This approach should only be used to make a bacterial diagnosis, not to determine the presence of a pocket infection. Entering an intact pocket should be avoided to avoid inoculation with bacteria.
During an extraction procedure, distal and proximal lead fragments, lead vegetation if present and generator pocket tissue should be sent for culture (Table 6).71 Gram stain is still encouraged and biomolecular methods are increasingly used and may be more specific. Culture media suggested are chocolate agar incubated in 5% CO2 for 48–72 h, MacConkey agar incubated for 48 h, blood agar in anaerobic condition for 48–72 h, and Sabouraud agar incubated for 5 days.72,73 A close collaboration with the local Microbiology Laboratory is important to increase diagnostic yield. In case of pus, but no growth after 3 days, consider slow-growing microorganisms including C. acnes and increase incubation duration. In addition to swabs, tissue samples and sonication for the recovery of bacteria from CIED leads and tissue, may be useful in patients with clinical signs of infection although the method merits further investigational study.68–70
Imaging
Echocardiography
Echocardiography should be the first imaging tool in the assessment of patients with CIED in order to identify lead vegetations and valvular involvement.59 Transthoracic- (TTE) and transoesophageal echocardiography (TEE) are both recommended in case of suspected CIED infections. While TTE better defines pericardial effusion, ventricular dysfunction, and pulmonary vascular pressure, TEE is superior for the detection and sizing of vegetations74 especially in the right atrium-superior vena cava area and in regions less well visualized by TTE. In the absence of typical vegetations of measurable size, both TTE and TEE may be false negative in CIED-related infective endocarditis. Lead masses in asymptomatic CIED carriers may be observed on TTE/TEE and do not predict CIED-related infective endocarditis over long-term follow-up.75,76 Therefore, once a lead mass is identified, careful clinical assessment to rule out either infection or non-bacterial lead-thrombotic endocarditis is needed, including serial TTE/TEE or additional imaging tests.
Intracardiac echocardiography (ICE) is effective and has a high sensitivity for the detection of vegetations in cardiac devices.77,78 Therefore, a vegetation seen with ICE may be considered a major criterion for diagnosis (Table 5). Recently, transvenous biopsy, guided by TEE, was shown to be useful to differentiate vegetation from thrombus.79
In patients with CIED infections treated with percutaneous lead extraction, a TTE before hospital discharge is recommended to detect retained segments of the pacemaker lead, and to assess tricuspid valve function, right ventricular function, and pulmonary hypertension. A TEE (and additional imaging tests) should be considered after percutaneous lead extraction in order to detect infected material, ghosts,80 and potential tricuspid valve complications, particularly in patients with persistent sepsis after extraction (Table 7). It is important to remember a normal echocardiography does not rule out CIED-related infective endocarditis.
Table 7.
Recommendations for diagnosis of CIED infections by imaging59
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| TTE is recommended as the first-line imaging modality in patients with suspected CIED-related IE |

|
O | 81 |
| A chest X-ray should be performed in all patients with suspected CIED infection |

|
E | |
| TEE is recommended in suspected CIED infection with positive or negative blood cultures, independent of TTE results before an extraction, to evaluate CIED infection and IE |

|
O | 74 |
| Repeat TTE and/or TEE within 5–7 days is recommended in case of initially negative examination when clinical suspicion of CIED-related IE remains high |

|
O | 81 |
| TEE should be performed in CIED patients with S. aureus bacteraemia |

|
O | 82 , 83 |
| ICE may be considered if suspected CIED-related IE, with positive blood cultures and negative TTE and TEE results |

|
O, E | 77 , 78 |
| [18F]FDG PET/CT scanning or radiolabelled WBC scintigraphy or contrast enhanced CT are recommended if suspected CIED-related IE, positive blood cultures, and negative echocardiography (attention in imaging interpretation early after device implant) |

|
O, M | 84 , 85 |
| [18F]FDG PET/CT should be performed in case of S. aureus bacteremia in CIED patients |

|
O, E | 86 ,87 |
| [18F]FDG PET/CT, radiolabelled WBC scintigraphy and/or contrast enhanced CT is recommended for identification of unexpected embolic localizations (i.e. lung embolism) and metastatic infections |

|
O, M | 84 , 88 , 89 |
| The identification of the infection portal of entry may be considered by [18F]FDG PET/CT and WBC imaging in order to prevent IE relapse |

|
O, E | 84 , 90 |
| Pulmonary CT angiography is recommended in patients with recurrent pneumonia |

|
O, E | 91 |
| In patients with CIED infection treated with percutaneous lead extraction, TTE/TEE before hospital discharge are recommended to detect presence of retained segments of pacemaker lead, and to assess tricuspid valve function, RV function, and pulmonary hypertension |

|
O | 80 , 92 , 93 |
In case of persistent sepsis after device extraction:
|

|
O, M | 84 , 94–97 |
| A multidisciplinary team (the Endocarditis Team) is recommended for evaluation of imaging results |

|
E | 98 |
[18F]FDG PET/CT, fluorine-18-fludeoxyglucose positron emission tomography/computerized tomography scanning; CIED, cardiac implantable electronic device; E, expert opinion; ICE, intracardiac echocardiography; IE, infective endocarditis; M, meta-analysis; O, observational studies; R, randomized trials; RV, right ventricular; TEE, transoesophageal echocardiography; TTE, transthoracic echocardiography; WBC, white blood cell count.
Radiolabelled leucocyte scintigraphy, positron emission tomography, and computerized tomography
Fluorine-18-fludeoxyglucose ([18F]FDG) positron emission tomography/computerized tomography (PET/CT) scanning and radiolabelled leucocyte (WBC) scintigraphy are complementary tools for the diagnosis of CIED-related infections and related complications in complex cases. Both imaging techniques provide additional diagnostic value, particularly in the subset of possible CIED infections, and may distinguish between early-onset superficial surgical site infection and a true generator pocket infection or differentiate between superficial and deep pocket infection. When patients only present with systemic infection without local findings at the generator pocket the diagnosis of device lead infection can be challenging and a [18F]FDG PET/CT is in this situation useful for the diagnosis of local infection [pooled specificity and sensitivity of 93% (95% CI 84–98%) and 98% (95% CI 88–100%), respectively, and AUC of 0.98 at ROC analysis].85,99 Mild inflammatory changes after device implantation usually do not extend beyond 6 weeks and are easily differentiated from infection after this period. White blood cell scintigraphy including single-photon emission tomography/computerized tomography (SPECT/CT) has high sensitivity and specificity for the detection and localization of CIED-related infections (94% and 100%, respectively, in the largest study).89 In case of CIED-related infective endocarditis, [18F]FDG PET/CT and WBC are very specific when tracer uptake is visualized (only if applied late after implantation), although a negative result does not completely exclude the presence of small vegetations with low metabolic activity (i.e. limited sensitivity and negative predictive value). Therefore, the diagnostic accuracy for lead infections is lower,99,100 with overall pooled sensitivity of 65% (95% CI 53–76%), specificity of 88% (95% CI 77–94%), and AUC of 0.861.
[18F]FDG PET/CT has the ability of whole-body evaluation, so has proven particularly useful for the identification of unexpected embolic localizations and metastatic infections,84,88 including mycotic aneurysms, spleen and lung embolisms, and spondylodiscitis (not brain emboli). This impacts the Duke criteria, the diagnostic certainty, and therapeutic management. Moreover, the identification of the infection entry site by PET/CT and WBC imaging is critical for the prevention of infective endocarditis relapse.90 Positron emission tomography/computerized tomography imaging may also contribute to mortality risk stratification assessment after lead extraction. Patients with definite CIED infection without pocket involvement on [18F]FDG PET/CT had unfavourable outcome, suggesting that the presence of an endovascular infection stemming from an unrecognized/distant site is associated with poor prognosis.101
Contrast-enhanced CT combined with PET may prove useful in selected patients. The addition of contrast-enhanced CT to standard [18F]FDG PET/CT protocol resulted in a high rate of reclassifications from ‘possible’ to ‘definite’ infective endocarditis, improving the overall diagnostic accuracy with or without the Duke criteria in a series of patients with suspected pulmonary embolism or CIED infections.84 Cardiac CT angiography may also add important remote information on vascular complications including mycotic aneurysm, arterial emboli, and septic pulmonary infarcts, which add to the diagnostic criteria and affect the overall treatment strategy. In addition, pulmonary CT angiography may be useful in patients with recurrent pneumonia.91 A wider use of contrast-enhanced CT is limited by the deleterious impact of contrast agents on kidney function particularly as the patients are exposed to nephrotoxic antibiotic therapy. An extensive description of the technical aspects and the interpretation criteria for multimodality imaging has recently been published.85
Multidisciplinary team (the Endocarditis Team) evaluations of imaging results are recommended and have been shown to significantly reduce the 1-year mortality,98 from 18.5% to 8.2%. Figure 2 shows the proposed diagnostic flow chart for the use of imaging in patients with suspected CIED infection.
Management of cardiac implantable electronic device infections: when, how, and where
Cardiac implantable electronic device removal
The key aspect to successful treatment of definite CIED infections is complete removal of all parts of the system and transvenous hardware, including the device and all leads (active, abandoned, epicardial as well as lead fragments) as well as vascular ports or permanent haemodialysis catheter.102,103 This treatment concept applies to systemic as well as localized CIED pocket infections.81 In a retrospective study of 416 patients with CIED infections, antibiotic therapy without device removal was associated with a seven-fold increase in 30-day mortality (hazard ratio 6.97, 95% confidence interval 1.36–35.60) in multivariate analysis.104
The timing of the extraction procedure should be without unnecessary delay after the diagnosis of CIED infection (Figures 2 and 3) and should take place at an experienced centre (Table 8). The performance of transvenous lead extraction within 3 days after hospitalization results in significantly lower in-hospital mortality and shorter hospitalizations in patients with CIED infections.114 It is important to note that despite correct treatment with CIED system explantation and adequate antibiotic therapy, the mortality in patients with systemic infection is significantly higher than in patients with local infection. Based on the results of the ELECTRa registry,2 systemic infection was identified as a predictor for increased all-cause mortality (OR 4.93, 95% CI 2.72–8.93, P < 0.0001).
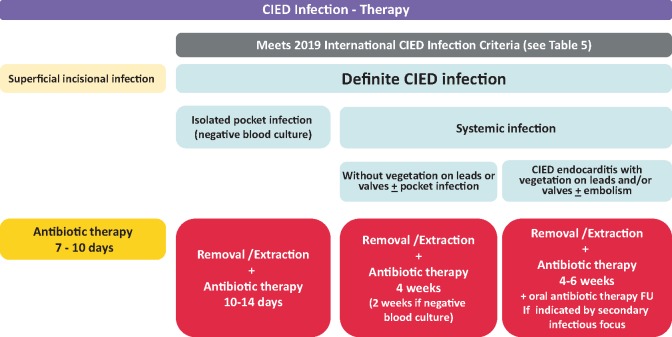
Figure 3.
Therapeutic strategies for patients with CIED infections. CIED, cardiac implantable electronic device; FU, follow-up; IE, infective endocarditis.
Table 8.
Recommendations for device and lead removal
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| In patients with definite CIED infection (systemic and local), complete device removal is recommended (including abandoned leads, epicardial leads, and lead fragments) |

|
O | 81 , 102 , 104 |
| After diagnosis of CIED infection, the device removal procedure should be performed without unnecessary delay (ideally within 3 days) |

|
O | 104 |
| The recommended technique for device system removal is percutaneous, transvenous extraction technique. Epicardial leads require surgical removal |

|
O | 105 |
| In patients with systemic infection and lead vegetations of approximately >20 mm, percutaneous aspiration of vegetations prior to and during transvenous lead extraction or alternatively surgical extraction may be considered |

|
O | 105–107 |
| After device removal, meticulous debridement of the generator pocket (complete excision of the fibrotic capsule and complete removal of all non-absorbable suture material) and subsequent wound irrigation with sterile normal saline solution is recommended |

|
E | 108 |
| Cultures of extracted CIED should be performed |

|
E, O | 66 |
The following wound closure methods after device removal and debridement of device pocket may be performed:
|

|
E | NA |
| Complete CIED removal is indicated in bacteraemia or fungaemia with S. aureus, CoNS, Cutibacterium spp., and Candida spp |

|
E | 109 |
| In bacteraemia with alpha- or beta-haemolytic Streptococcus spp. and Enterococcus spp., a complete CIED removal may be performed as first-line treatment or in case of recurrent/continued bacteraemia despite appropriate antibiotic therapy as a second step therapy |

|
E | 110 |
| In case of bacteraemia with non-pseudomonal/Serratia Gram-negative bacteria or Pneumococcus spp., CIED removal should be performed in the case of recurrent/continued bacteraemia despite appropriate antibiotic therapy when there is no other identifiable source for recurrence or continued infection |

|
E | 14 , 72 , 111 , 112 |
| Complete CIED removal is recommended in patients with infective endocarditis with or without definite involvement of the CIED system |

|
E | 113 |
| Blood cultures should be taken 48–72 h after removal of an infected CIED |

|
E | 19 |
CIED, cardiac implantable electronic device; E, expert opinion; M, meta-analysis; NA, not available; O, observational studies; R, randomized trials.
When applicable, percutaneous transvenous extraction techniques are the methods of first choice (Table 8), since major complications and mortality at 1 and 12 months in patients undergoing transvenous lead extraction techniques is significantly lower compared to open surgical approaches.105,115 Transvenous extraction procedures are even preferred in the presence of lead vegetations with a diameter of more than 10 mm (Table 8). Small case series have reported good short-term outcomes of transvenous lead extraction procedures in patients with large lead vegetations despite a high percentage of pulmonary embolism. However, long-term outcomes of such patients remain unclear.116,117 In patients with vegetations larger than ∼20 mm open surgical extraction may be considered.59,81 Even with transoesophageal echocardiography the vegetation size may be difficult to assess. Apart from size, the friability of the vegetation should be taken into consideration when planning a procedure. A promising concept in patients with systemic CIED infection and very large lead vegetations is percutaneous aspiration of lead vegetations with the help of a veno-venous extracorporeal circuit with an in-line filter.106,107 The goal of this treatment is to reduce the overall ‘vegetative’ burden and the risk of embolization of infectious material into the pulmonary circulation, which may be a source of ongoing septic complications.
In case of infections of CIED systems with epicardial leads, complete removal of such leads is recommended in case of definite involvement based on individual risk–risk-analysis (operative risk of epicardial lead removal vs. infection-related mortality risk).115 In case of localized pocket infection of a CIED system without definite involvement of the distal portion of an epicardial lead, it is reasonable to leave the distal portion of the epicardial lead in place by cutting the lead through a separate incision away from the device pocket and removing the proximal part of the lead through the pocket.81 [18F]FDG/PET/CT scan may prove helpful in assessing such special situations.
In cases of occult bacteraemia or fungaemia, the results of microbiological examination influence further therapy. Complete CIED removal is indicated in bacteraemia or fungaemia with S. aureus, CoNS, Cutibacterium spp., and Candida spp. In bacteraemia with alpha- or beta-haemolytic Streptococcus spp. and Enterococcus spp. complete CIED removal may be carried out as first-line treatment or as a second step in case of recurrent/continued bacteraemia despite appropriate antibiotic therapy. In case of bacteraemia with non-pseudomonal/Serratia Gram-negative bacteria or Pneumococci an appropriate antibiotic therapy is indicated first. In case of recurrent/continued bacteraemia, complete CIED removal should be performed in cases when no other identifiable source for recurrence or continued infection is found.14,72,102,111,112 Complete CIED removal is furthermore indicated in patients with infective endocarditis without definite involvement of the CIED system.113
After device and lead removal a meticulous debridement of the device pocket with complete excision of the fibrotic capsule as well as removal of all non-absorbable suture material and subsequent wound irrigation with sterile normal saline solution is crucial. Irrigation with antibiotic solution does not offer any significant advantage over irrigation with saline solution.108 Wound closure concepts may be primary closure with or without the use of a drain or alternatively delayed closure utilizing negative pressure wound therapy.
Cardiac implantable electronic device patients with superficial wound infections early after implantation, device exchange or revision surgery should not undergo device and lead removal. Superficial infections are confined to the skin and the subcutaneous tissue without involvement of any parts of the CIED system. The differentiation between a superficial and a pocket infection can be a clinical challenge. Therefore, it is important to closely watch patients who are under suspicion of having a superficial infection. In such patients, an oral antibiotic therapy (7–10 days) is reasonable.65
Antimicrobial therapy including long-term suppressive therapy
Definitive treatment of CIED infection is early and complete removal of all parts of the system and antibiotic therapy is to be seen as a complement to treat associated systemic infection and to cure the remaining infection in native tissues.103 Randomized controlled studies to guide antibiotic choice in CIED infections are lacking and recommendations are based on cohort studies, case series, expert opinion, and pharmacological considerations.19,59,65 Access to timely device removal influences antibiotic therapy and treatment regimens also differ between countries depending on the prevalence of MRSA, other differences in antibiotic resistance patterns, and access to specific antibiotics including newer substances. Antibiotic combination therapy with rifampicin targeting biofilm-associated staphylococcal infection is not recommended when the device will be removed unless a concomitant foreign body associated infection, i.e. a prosthetic valve endocarditis that is not amenable to replacement surgery, is present. Antibiotic treatment alone is not recommended for CIED infections but individual patients may not be candidates for device removal. Successful salvage therapy has been reported in a minority of patients.103 Long-term suppressive antibiotic therapy is also used in selected cases.118
Antibiotic treatment recommendations including empirical choices for the three major categories of CIED infections, superficial incisional infection, isolated device pocket infection, and systemic infections, are summarized in Table 9. Systemic infections are further divided depending on presence of positive blood cultures and vegetations on leads and/or valves.65
Table 9.
International consensus recommendations for antibiotic therapy including long-term suppressive therapy
| Consensus statement | Statement class | Scientific evidence coding | References | |
|---|---|---|---|---|
| Superficial incisional infection | ||||
|
|

|
O, R | 19 , 65 |
| Isolated pocket infection (negative blood cultures) | ||||
|
|

|
O, R | 19 , 59 , 65 |
| Systemic infections | ||||
| Without vegetation on leads or valves ± pocket infection | ||||
|
|

|
O, R | 19 , 59 , 65 , 81 |
| CIED endocarditis with vegetation on leads and/or valves ± embolism | ||||
|
|

|
O, R | 59 |
| Bacteraemia in a CIED patient without signs of pocket infection or echocardiographic evidence of lead or valve involvement | ||||
| According to pathogen specific treatment guidelines, see text |

|
O, R | 119 , 120 | |
| Attempted salvage therapy and long-term suppressive therapy | ||||
| I.v. antibiotics as in prosthetic valve endocarditis for 4–6 weeks Stop antibiotic therapy under close follow-up or continue individualized long-term suppressive oral therapy, see text |

|
E | 103 , 118 | |
Treatment regimens differ between countries depending on prevalence of MRSA and other circumstances—see text. Dosage recommendation needs to be adjusted for kidney function.
For patients with normal renal function.
d, day; E, expert opinion; H, hour; i.v., intravenous; M, meta-analysis; MRSA, methicillin-resistant Staphylococcus aureus; O, observational studies; od, once daily; p.o., per oral; R, randomized trials.
For superficial incisional infection, a wound culture before initiation of antibiotic treatment is recommended (Table 9).
For isolated pocket infections empirical i.v. therapy is recommended after blood cultures have been obtained (Table 9, Figure 3). Definitive treatment should be given according to culture result with an appropriate antibiotic with a narrow spectrum, if possible a betalactam antibiotic. Combination therapy is not needed. A switch to oral treatment after device removal is reasonable since the remaining infection only involves skin and soft tissue, but evidence-based recommendations are lacking. In pocket erosion with minimal inflammation, delayed antibiotic therapy until after device removal and pocket cultures should be considered.
For pocket infection with positive blood culture but without vegetation on leads or valves, the definite treatment follows recommendations given above but the systemic involvement makes a switch to an oral antibiotic regimen inappropriate (Table 9, Figure 3). Shorter post-extraction treatment duration is considered possible by some experts.19
For blood culture positive CIED endocarditis with vegetation on lead or valve the recommendations follow guidelines for infective endocarditis (Table 9).59 If the TEE performed after device removal shows no signs of valve vegetation (i.e. isolated lead vegetation), the follow-up blood cultures are negative, the clinical improvement is good and there are no pulmonary abscesses, treatment duration for 2 weeks post-device extraction can be sufficient but total treatment duration should not be shorter than 4 weeks (Figure 3).
For bacteraemia in a CIED patient without signs of pocket infection or echocardiographic evidence of lead or valve involvement, the antibiotic treatment follows general recommendations. Due to the risk of undetected device infection, device removal should be considered even in the absence of vegetations, in case of infection with specific pathogens or relapsing bacteraemia without other source, but randomized studies are lacking.119 The addition of rifampicin is not recommended in patients with S. aureus bacteraemia but can be considered in the presence of concomitant non-removable foreign body.120,121 For S. aureus, CoNS, Cutibacterium spp., and Candida spp., CIED removal is generally recommended. With viridans group and beta-haemolytic Streptococcus spp. or Enterococcus spp., device removal should be considered as well as prolonged i.v. treatment (4 weeks). Even though Gram-negative bacteria are capable of secondary seeding of a device,72 concomitant CIED infection is uncommon in non-pseudomonal/Serratia Gram-negative or pneumococcal bacteraemia, and device removal is generally not needed.14,111,112
For attempted salvage therapy if complete device removal is not possible, long-term suppressive therapy with i.v. antibiotic following recommendations in prosthetic valve endocarditis for 4–6 weeks is reasonable (Table 9, Figure 3). If oral suppressive therapy is planned, antibiotic therapy should be chosen according to culture results but evidence-based recommendations cannot be made.103,118 In methicillin-sensitive staphylococci, oral flucloxacillin is considered an option by some experts but is not used by others due to low oral bioavailability. In methicillin-resistant S. aureus or CoNS, oral trimethoprim-sulfamethoxazole, clindamycin, or doxycyclin (if sensitive) are alternatives. Linezolid is not suitable for long-term treatment. Rifampicin and fusidic acid are not suitable as single therapy. A combination of suppressive therapy is generally not preferred. The treatment duration needs to be individualized.
Preventive strategies after cardiac implantable electronic device implantations, new re-implantations, and alternative novel devices
Preventive strategies after cardiac implantable electronic device implantations
Since the removal or extraction of CIEDs is usually mandated if infection occurs, prevention of infection is a key goal. While primary prevention measures, including prophylactic antibiotic therapy,21 appropriate infection control measures, and careful surgical technique, are well established and highly recommended, data supporting the benefits of secondary prophylaxis are relatively scarce and controversial. Early follow-up in a clinical setting and thorough patient educational programs should be conducted for early identification of CIED-related infectious complications, including video consultations for wound inspections. A short period of treatment may prevent progression, but may also mask a pocket infection, delay appropriate treatment, or expose the patient to unnecessary medical therapy.
At present, there is no convincing evidence that microorganisms associated with invasive medical procedures cause infection of non-valvular vascular devices at any time after implantation (Table 10). Therefore, antibiotic prophylaxis is not routinely recommended for CIED patients who undergo dental, respiratory, gastrointestinal, genitourinary, or cardiac procedures. Secondary prophylaxis is only recommended for patients when they undergo incision and drainage of infection at other sites or replacement of an infected device.134
Table 10.
Recommendations for preventive strategies after device implantation and for new re-implantations including alternative novel devices
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| After device extraction, re-assessment of the indication for re-implantation is recommended |

|
O | 38 , 122 |
| Whenever possible, re-implantation may be avoided or delayed until symptoms and signs of systemic and local infection have resolved |

|
O | 38 , 123 |
| A temporary pacemaker with ipsilateral active fixation strategy may be considered in pacemaker-dependent patients requiring appropriate antibiotic treatment before re-implantation |

|
O | 124–127 |
| Preferred access sites for replacement device are the contralateral side, the femoral vein, or epicardially |

|
E, O | 38 , 128 , 129 |
| Temporary pacing in patients who are not pacemaker dependent |

|
O | 28 |
| Replacement device implantation ipsilateral to the extraction site |

|
E | 38 |
| Alternative novel devices as LPM and S-ICD may be considered in selected patients with high infective risk or in patients in whom these devices are considered better options after an CIED infection |

|
O | 129–133 |
CIED, cardiac implantable electronic device; E, expert opinion; LPM, leadless pacemaker; M, meta-analysis; O, observational studies; R, randomized trials; S-ICD, subcutaneous implantable cardiac defibrillator.
Re-implantations
All established CIED infections, systemic or localized, mandate complete hardware removal and no part of the removed CIED should be re-implanted. This also applies to the venous access sheath used for percutaneous removal, which should not be used for re-implantation of a new system. Central and peripheral lines and any other removable catheters should also be changed at this time, where feasible.
The indication for re-implantation should always be re-evaluated after a CIED removal.38,122 A different device than the previous one may be the most optimal choice or there may be no absolute indication for re-implantation at the time of lead extraction. There are no randomized trials guiding appropriate timing of re-implantation and therefore such decision must be individualized. Re-implantation should be delayed until signs and symptoms of local and systemic infection have resolved or postponed until blood cultures are negative for at least 72 h after the extraction if feasible (Table 10).38,123,135 In pacemaker-dependent patients, it seems reasonable to use temporary pacing until symptoms and signs of systemic infection have resolved before implanting a permanent device. An active-fixation lead ipsilaterally implanted, albeit preferably not through the vein from which the infected lead was extracted, and connected to an externalized pacemaker could be the best strategy in order to safely delay re-implantation124–127 by keeping the infected extraction side for temporary pacing and preserving the contralateral side for definitive device re-implantation.57,81,128 Epicardial lead placement has been used for decades as the only reliably strategy for patients at very high risk of re-infection.
Alternative novel devices
Leadless pacemakers (LPM) and S-ICD are excellent alternatives to traditional CIEDs. Currently, two self-contained right ventricular pacemakers implanted by using a femoral percutaneous approach have been developed: Micra™ Transcatheter Pacing System (TPS; Medtronic, Minneapolis, MN, USA) and Nanostim™ Leadless Cardiac Pacemaker (LCP; St. Jude Medical, Sylmar, CA, USA), although only the former is currently available for clinical use. It can pace the right ventricle with a rate response technology and may represent a valid and alternative solution, if indication is appropriate. No infection occurred after a short/mid-term follow-up.129 In selected high-risk patients, the risk of infection with LPM appears low.41 The device also seems safe and feasible in patients with pre-existing CIED infection and after extraction of infected leads.130,136
Similarly, S-ICD has proven to be safe and effective in many clinical trials in detecting, discriminating, and terminating potentially life-threatening ventricular arrhythmias, even if it is not able to deliver anti-tachycardia pacing and can only provide post-shock ventricular pacing at 50 b.p.m. for 30 s. In selected patients with no need for pacing, ATP or CRT, the re-implantation of a S-ICD significantly reduced the risk of new infections while still providing an effective and safe defibrillation system.132,133,137 While they do not offer complete protection against infection, their removal is much simpler and most often does not result in a life-threatening systemic infection.138 While randomized trial data are still forthcoming, data from the 985 patients enrolled into the European EFFORTLESS Registry found an infection rate (requiring device removal) of 2.4% over 3 years of follow-up.139
In the near future, the LPM and the S-ICD (Boston Scientific) are expected to integrate wireless intrabody communication between devices to co-ordinate pacing and antitachycardia therapy delivery.133
For patients with a high risk of sudden cardiac death on short-term and no need for pacing a wearable defibrillator (LifeVest, Zoll) is an option as a bridge to re-implantation.
Prognosis, outcomes, and complications of cardiac implantable electronic device infections
Cardiac implantable electronic device infection has an in-hospital or 30-day mortality of 5–8%133,140,141 including mortality from lead extraction, usually reported as ∼0.5%,2 but which is principally related to the complications of ongoing sepsis. The mortality is higher for patients with significant co-morbidities (e.g. cardiac failure, renal failure, corticosteroid use), with CIED endocarditis rather than pocket infection,114 and for patients who do not undergo complete removal of CIED hardware.118 In-hospital morbidity also comprises the complications of lead extraction, usually reported as 2–3%, including emergency thoracotomy for perforation, arterio-venous fistulae, and tricuspid valve damage; septic pulmonary emboli (∼1%); arrhythmias; and ongoing sepsis.2 Following device removal, complications can arise from the re-implantation of a new device, particularly recurrent infection, although uncommon provided appropriate antibiotic treatment and timing of the new implant.
Patients who do not have complete removal of hardware, particularly because they are considered too frail, have a very high mortality in-hospital and over the months following discharge.118,142 A delay in device removal also leads to a worse prognosis.114
The long-term mortality in patients following CIED infection is up to 1.5–2.4 times the mortality rate of non-infected patients,140,143 which is 6–15% at 1 year and 14–33% at 3 years, the range of estimates reflecting the age and co-morbidities of the patients being studied. Patients with infective endocarditis144,145 and females have a higher long-term mortality rate than males,146 when adjusted for co-morbid factors. The presence of end-stage renal failure confers a particularly poor prognosis.147 However, it is not clear whether the higher long-term mortality is due to the CIED infection itself, or to the presence of poor prognostic factors in the patients who develop CIED infection (e.g. cardiac failure, renal failure, coagulopathy, corticosteroid use, diabetes), or whether a poor outcome reflects inadequate management. There is some indication that patients who have been successfully treated (‘cured’) with complete removal of hardware and a full course of antibiotics may have a similar prognosis to patients who have never been infected.148,149
Special considerations to prevent device-related infections (elderly, paediatrics, adult with congenital heart disease)
Certain patient populations pose additional risks and considerations for infection prevention. One of these populations are the elderly patients needing a pacemaker or ICD.150,151 In several studies, age per se is not an independent predictor of infection when adjusted for other co-morbidities, such as diabetes, renal failure, heart failure, malignancy, and pulmonary disease.5,22,28,152,153 Frailty, common in the elderly, is associated with worse cardiovascular outcomes.154,155 Cardiac implantable electronic device patients with decreased mobility and decreased activity are more likely to be frail.156 In a study of nearly 84 000 patients followed in the National Cardiovascular Device Registry-ICD (NCDR-ICD), combinations of frailty with other known risk factors for CIED infections were predictive of higher mortality.155 The elderly are often at greater risk of device erosion due to the loss of subcutaneous tissue and muscle mass.81,157 Using a sub-muscular approach may provide better protection against erosion in appropriate patients. There are several surgical approaches to the sub-muscular space and implanting physicians should be familiar with these.158 As in all patients, careful attention to haemostasis is important, as haematoma is a consistent risk factor for infection.7,24
Younger age also has been associated with a higher risk of CIED infection. In a study of 46 299 patients enrolled in the Danish Pacemaker Register, 2499 (5%) of patients were under the age of 20 years. In multivariate analysis, age under 20 years was found to have a 40% higher risk for infection (HR 1.41, 95% confidence interval 0.83–2.38).3 The higher risk of infections may be due to the total number and complexity of device-related procedures that a young person is exposed to over the course of their life.152,159,160 Colonization of the pocket may lead to higher rates of infections with subsequent generator replacements.161 The complication rates of procedures involving lead revisions or replacements is reported to be at least twice higher than that of de novo implants.21,22,28,162 In one study of 497 children receiving pacemakers over a 20-year period with median follow-up of 6 years, the lead failure rate was 15%, which occurred in 23% of the patients. Over a quarter (28%) of the patients experienced multiple failed leads. Risk of lead failure was associated with age <12 years at implant, congenital heart disease (CHD), and epicardial lead placement. The reported infection rate after lead replacement in this study was 1.9%.163
Data from the NCDR-ICD note low (0.2%) acute infection rates in adults with congenital heart disease (ACHD) patients.164 As in adults, most infections in paediatric ages and CHD present after longer-term follow-ups and appear to be in a similar range as that reported in adults (∼1–5% serious infections).3,145,151,152,159,165,166 In one retrospective registry study of 443 ICD paediatric or CHD patients, the 30-day and longer than 30-day cumulative infection rate was 4.9%.165 While lead extraction is reported to have high success in the paediatric and CHD population, nevertheless the serious consequences of CIED-related endocarditis and need for full device system extraction are not trivial.159,167
The approach to device implantation poses special challenges in children and CHD patients. Due to small size, abnormal anatomy, and restricted venous access, unusual surgical approaches may be required.168–173 Epicardial pacing leads and submuscular abdominal positions for the generator are used in the very young with ICD high voltage leads placed in the posterior-lateral pericardial space. As the children grow, transition to transvenous PM and ICD systems can occur if venous access is available. The submuscular (subpectoral) approach for generator placement has been preferred due to smaller size and/or cosmetic reasons.174 Regardless of specific approach, the young paediatric, CHD, and ACHD patients face a lifetime of device system revisions often resulting in the need for alternative approaches. An alternative approach for older children needing an ICD is the S-ICD. The absence of need for transvenous leads may decrease device-related complications including infection. A report of pooled data from the EFFORTLESS registry combined with the patients enrolled into the US FDA IDE trial showed that no infections occurred in the 19 patients with CHD (age 12–65) compared with 1.5% system infections in the remaining 846. Overall complications were similar (10.5% and 9.6%, respectively) over a follow-up about 2 years.175 In a report from the Alliance for Adult Research in Congenital Cardiology, 21 patients from 7 centres with ACHD received a S-ICD. At 14 months of follow-up, a single infection was identified which did not require device removal.176 Several additional reports have demonstrated favourable use of the S-ICD in these patients.177–179 Pacemakers that are programmed dedicated bipolar pacing are compatible with the S-ICD system and the combination has been reported in CHD.180
Recommendations for prevention of infections related to device implantations in elderly, paediatric patients and patients with ACHD are outlined in Table 11.
Table 11.
Recommendations for prevention of infections related to device implantations in elderly, paediatric patients, and in adults with congenital heart disease
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| Implanting physicians should be aware of the higher CIED infection risks in frail and elderly patients. Submuscular position of PM or ICD generators is recommended in selected elderly patients with limited subcutaneous tissue to prevent device erosion |

|
O | 158 |
| Implanting physicians should be skilled in multiple and alternative surgical approaches performed in paediatric, congenital heart disease, and ACHD patients related to a higher risk of CIED infection due to multiple procedures, lead addition and revisions, and upgrade procedures |

|
M, O | 168–172 |
| The entirely S-ICD should be considered as an alternative to transvenous or epicardial approaches in the older child, patients with congenital heart disease, and those with limited or no venous access. Patients with a bradycardia indication, anti-tachycardia pacing, or cardiac resynchronization therapy requirements are not appropriate candidates |

|
O | 175–179 |
ACHD, adults with congenital heart disease; CIED, cardiac implantable electronic device; ICD, implantable cardiac defibrillator; PM, pacemaker; S-ICD, subcutaneous implantable cardiac defibrillator; R, randomized trials; O, observational studies; M, meta-analysis; E, expert opinion.
Minimum quality requirements concerning centres and operator experience and volume
Data on association between centre volume and operator experience and volume and the occurrence of CIED infections are scarce and heterogeneously reported. Some studies date back to the pre-antibiotic era, all are observational in nature, and choice of predictors, statistical adjustments and CIED infection definitions vary widely as do follow-up periods. The recommendations given on centre and operator volumes are approximations based on observational data and expert consensus.
For every surgical procedure a learning curve exists during which complications are expected more frequently. For implantation of cardiac pacemakers, an operator experience <100 procedures was associated with higher risk of infection in the pre-prophylactic antibiotic era.181,182 Less than 100 procedures were also associated with a higher risk of any complication.183 Pocket haematoma, known to be associated with higher infection risk, was more common in patients implanted by operators with <100 procedures experience, and haematoma requiring re-intervention were more frequent with operators experience <50 procedures.184 Based upon these reports, it seems reasonable to recommend close supervision of operators with less than approximately 100 procedures experience.
Several large observational studies have reported complication risk for different operator volumes. For ICD implantations, operator volume <29 procedures per year was associated with adjusted odds ratio for infection of 2.47 (95% CI 1.18–5.17) compared to higher volume operators.185 Notably, more high-volume operators also had higher volume of pacemaker implantations.185 Considering any complication, operator volume <60 procedures per year was associated with a highly increased risk of complications [hazard ratio 10.4 (1.32–82.14)] in data from the Ontario ICD database.186 For cardiac pacemakers, operator volume >40 procedures per year was associated with fewer complications.187 From a Danish nationwide cohort study of CIED procedures, annual operators volume <50 procedures was associated with a 1.9 (1.4–2.6) adjusted risk ratio of any complication, and higher risk of CIED infection (1.7% vs. 0.5%, P = 0.02).188 Therefore, an annual minimum operator volume of approximately 50 CIED procedures is recommended.
For centre volume, data are even less solid. Centres with lower implantation volume (<250 per year) were reported with higher infection risk for replacement procedures.7 In historical data from the Danish Pacemaker Registry, infection rates for cardiac pacemakers were similar between high-volume university centres and lower volume centres (<200 procedures per year).3 From a prospective Danish nationwide cohort, centres with implant volume >750 per year had the lowest risk of any complication, while infections did not differ between centre volumes.188 However, the smallest centres in this analysis had up to 249 implants per year. In the Dutch FOLLOWPACE registry, including centres with mean annual volumes from 53 to 220 procedures, no association between centre volume and risk of any complication was observed,189 while a large German nationwide analysis clearly indicated an inverse relationship between risk of any early surgical complications and centre procedure volume.190 Therefore, any specific recommendation for minimum centre volume will be arbitrarily and consensus based. However, a centre needs ≥2 implanters, each performing ≥50–60 procedures. Allowing for training, a minimum centre volume of approximately 150 procedures per year is therefore recommended.190 The recommendations on minimum quality requirements concerning centres and operator experience and volume are outlined in Table 12. Every centre should monitor and report local infection rates to a database.
Table 12.
Recommendations on minimum volume requirements of cardiac implantable electrical device (CIED) procedures for centres and operators
| Consensus statement | Statement class | Scientific evidence coding | References |
|---|---|---|---|
| Operators with less than approximately 100 CIED procedures experience should work under close supervision of more experienced operators |

|
O, E | 181–184 |
| An annual minimum operator volume of approximately 50 CIED procedures is recommended for all operators |

|
O, E | 185–188 |
CIED, cardiac implantable electrical device; E, expert opinion; M, meta-analysis; O, observational studies; R, randomized trials.
Health economics for cardiac implantable electronic devices infections and strategies to reduce costs
The incidence of CIEDs infections is increasing at a faster rate as compared with device implantation rates.4,153,191 This has important implications for the healthcare systems in view of induced healthcare costs, related to a hospitalization usually of 2–4 weeks, with wide use of antibiotics and need for procedures for device removal and lead extraction, as well as CIED re-implantation.4,6,192 Estimates of the costs of CIED infection are limited, with reported values of €20 623–€23 234 in France, €36 931 (£30 958) in the UK, and values ranging between €15 516 and €337 886 ($16 651 to $362 606) in the USA, with the wide range related to treatment intensity and complexity.6,11,193 For interpreting these estimates, it is noteworthy to consider that any added day of in-hospital stay has huge costs, ranging from $476–835 in European countries to $4287 (on average) in the USA.194
In view of these costs, it is critical that the reimbursement tariffs properly cover the costs due to management of CIED infection patients in all the involved centres. In Europe, reimbursement practices usually are based on Diagnosis Related Groups and show an important variability for device procedures,195 and if that tariff is inadequate, the reimbursement policy can ‘interfere’ with physician choices in most complex patients with a substantial risk of suboptimal care.196
Health technology assessment is the specific tool for a comprehensive approach to this complex issue, involving all the stakeholders.197,198 Large national and international registries are of crucial importance for analysing the pattern of referral, the results of lead extraction and the outcomes, in the perspective of optimization of the chain of care, involving many stakeholders, in a ‘hub and spoke’ organization system which should guarantee expert care, coupling effectiveness with appropriate use of resources.2,196
Divergent recommendations from different societies
Strategies for preventing and managing CIED infections vary widely, and the evidence to guide practice is limited. Until now, guidelines on how to prevent, diagnose, and treat CIED infections were published only by the American Heart Association (2010)65 and by the British Society for Antimicrobial Chemotherapy, British Heart Rhythm Society, British Cardiovascular Society, British Heart Valve Society, and British Society of Echocardiography (2015).19
In addition, guidelines for the management of infective endocarditis were published in 2015 by the European Society of Cardiology (ESC)59 and by the American Heart Association.199 Recently, the Heart Rhythm Society (HRS) developed a Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction providing practical clinical guidance in the broad field of lead management, including lead infectious process.81 A summary of the main divergent recommendations across these five guidelines is available in Table 13.
Table 13.
Recommendations/guidelines published by different societies on management of cardiac implantable electronic device infections
| Guidelines content | Recommendations | AHA65 2010 | BHRS19 2015 | ESC59 2015 | AHA199 2015 | HRS81 2017 | EHRA 2019a |
|---|---|---|---|---|---|---|---|
| Diagnosis | |||||||
| Transoesophageal echocardiography | TEE should be used for diagnosis since it has higher sensitivity in establishing intravascular related CIED infection than TTE | ✓ | ✓ | ✓ | ✓ | ✓ | 7 |
| [18F]FDG PET/CT/scintigraphy with radiolabelled WBC | [18F]-FDG PET/CT/scintigraphy with radiolabelled WBC should be used as an additive diagnostic tool | NA | Only for research | ✓ | NA | ✓ | 7 |
| Blood cultures | Blood cultures should be taken 48–72 h after removal of infected CIED | NA | ✓ | NA | NA | NA | 6 |
| Generator pocket tissue/lead | Percutaneous aspiration of generator pocket should not be performed | ✓ | NA | NA | NA | NA | 6 |
| Generator pocket tissue/lead | Tissue should be excised from pocket site and sent for culture | NA | ✓ | NA | NA | NA | 6 |
| Radiography | Chest X-ray should be performed if suspected CIED infection | NA | ✓ | NA | NA | NA | 7 |
| ceCT/CT pulmonary angiography | ceCT or CT pulmonary angiography should be considered when CIED infection is suspected and echocardiography is non-diagnostic | NA | ✓ | NA | NA | NA | 7 |
| Treatment—CIED management | |||||||
| Early post-implantation inflammation | In superficial or early inflammation, the CIED can initially be left in situ. | ✓ | ✓ | NA | NA | NA | 8 |
| Isolated pocket infection/erosion | The CIED must be removed completely within 2 weeks after diagnosis | ✓ | ✓ | NA | NA | NA | 8 |
| CIED lead infection | Complete device system must be removed in CIED lead infection. | ✓ | ✓ | NA | NA | ✓ | 8 |
| CIED infective endocarditis | Complete removal is mandatory in CIED infective endocarditis | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| Occult bacteraemia | Complete device removal is recommended in occult bacteraemia | ✓ | NA | NA | NA | ✓ | 8 |
| Device reimplantation | New transvenous lead implant should be postponed if possible, to allow a few days or weeks of antibiotic therapy | ✓ | ✓ | ✓ | NA | ✓ | 10 |
| Device reimplantation | The replacement device implantation should not be ipsilateral to extraction site. Preferred locations are contralateral side, iliac vein, or epicardial | ✓ | ✓ | NA | NA | ✓ | 10 |
| Treatment—antibiotic strategy | |||||||
| Early post-implantation inflammation | In early post-implantation inflammation, the use of antibiotic therapy should be determined on a case by case basis | NA | ✓ | NA | NA | NA | 9 |
| Uncomplicated pocket infection | In uncomplicated pocket infection, empirical antibiotic therapy can be used | NA | ✓ | NA | NA | NA | 9 |
| Complicated pocket infection | Duration of antibiotic therapy should be 10–14 days after CIED removal for pocket-site infection | ✓ | NA | NA | NA | NA | 9 |
| Complicated pocket infection | Antibiotic treatment options and duration depend on echo findings; if no native valve involvement, treat as uncomplicated generator pocket infection | NA | ✓ | NA | NA | NA | 9 |
| CIED lead infection | Duration of antibiotic therapy should be at least 14 days after CIED removal for bloodstream infection | ✓ | NA | NA | NA | NA | 9 |
| CIED infective endocarditis | The duration of antibiotic therapy should be at least 4–6 weeks for complicated infection | ✓ | ✓ | NA | ✓ | NA | 9 |
| Antibiotic prophylaxis | Systemic antibiotic prophylaxis should be used prior to CIED implantation | ✓ | ✓ | NA | NA | NA | 4 |
| Team/reference centre | Complicated infective endocarditis should be referred early and managed in a reference centre with immediate surgical facilities (‘Endocarditis Team’) | NA | NA | ✓ | NA | NA | 7 |
18F-FDG PET/CT, fluorine-18-fludeoxyglucose ([18F]FDG) positron emission tomography-computerized tomography (PET/CT) scanning; CIED, cardiac implantable electronic device; NA, not available; TEE, transoesophageal echocardiography; TTE, transthoracic echocardiography; ✓, agreed recommendation.
The number refers to the table for the recommendation in the present document where the particular subject was addressed.
General definitions and minimal requirements of variables in scientific studies and registries
Cardiac devices registries have been used in many centres around the world; however, a standard dataset definition for patients with CIED infection remains unavailable. A document dedicated to this topic regarding lead extractions has been published.57 Adopting standardized data elements and a standard terminology is arguably the key to facilitate the exchange of data across studies and to promote interoperability between different electronic health records systems.200–202 In addition, standardized outcomes measurement will probably open up new possibilities to compare performance globally, providing the medical community with real-world evidence to improve healthcare for patients. A minimum dataset of variables is detailed in Table 14 to serve as a guide when designing CIED infection registries both in the scientific and clinical settings. The necessity of capturing individual parameters will depend upon the purpose of the study/registry.
Table 14.
Minimal study variables for scientific studies and registries
| Variable group | Data field | Value domain |
|---|---|---|
| Hospital characteristics | ||
| Lead extraction reference centre | Yes/No | |
| Device implantation volumes | Numbers/year | |
| Demographic factors | ||
| Age | Date of birth | |
| Gender | Sex at birth | |
| Race | Ethnicity per country | |
| Intravenous drug abuse | Yes/No | |
| Educational level | Level of schooling completed | |
| Baseline clinical factors | ||
| Comorbidities | Chronic kidney disease | Yes/No |
| Diabetes mellitus | Yes/No | |
| Heart failure | Yes/No | |
| Valve prosthesis | Yes/No | |
| Cancer | Yes/No | |
| HIV infection | Yes/No | |
| Medications | Oral anticoagulants | Yes/No |
| Corticosteroids | Yes/No | |
| Immunosuppressant drugs | Yes/No | |
| Specific risk factors | Percutaneous catheters for various indications (infusion, PM, haemodialysis) | Yes/No |
| CIED characteristics | Type of CIED | |
| Pacemaker DDD, SSI | ||
| ICD VR | ||
| ICD DR | ||
| CRT-P | ||
| CRT-D | ||
| ICD-subcutaneous | ||
| Leadless PM | ||
| Pulse generator pocket location | Pectoral/abdominal | |
| Lead access | Transvenous/epicardial/subcutaneous/hybrid | |
| Date of CIED implantation | YYYY-MM-DD or estimated | |
| Total leads number | (number) | |
| Abandoned leads | Yes/No | |
| Leads on both sides of the chest | Yes/No | |
| Prior treatments | ||
| Last CIED procedure | Initial implantation/generator replacement/lead revision/upgrade | |
| Date of infection diagnosis (time between last CIED procedure and current infection episode) | YYYY-MM-DD or estimated | |
| Abandoned leads | None/transvenous/epicardial | |
| Admission related to current infection episode | ||
| Date of admission | YYYY-MM-DD | |
| Symptoms—fever or hypotension | Yes/No | |
| Signs of generator pocket infection | Yes/No | |
| Signs of systemic infection | Yes/No | |
| Septic shock | Yes/No | |
| Diagnostic methods | ||
| Blood cultures | Microbial pathogens | |
| TE echocardiogram findings | CIED-related, RA, or RV mass | |
| Tricuspid vegetation | ||
| Left sided vegetation | ||
| Duke-criteria diagnosis | Definite CIED-related infection | |
| Suspected CIED-related infection | ||
| [18F]FDG PET/CT findings | CIED-related glycolytic activity | |
| Absence of glycolytic activity | ||
| Final diagnosis | Early post-implantation incision inflammation | |
| Uncomplicated pocket infection | ||
| Complicated pulse generator pocket infection | ||
| CIED-lead infection | ||
| CIED-associated infective endocarditis | ||
| Treatment | Antibiotic therapy | |
| Initial date of antibiotic therapy | YYYY-MM-DD | |
| Empirical antibiotic therapy | Yes/No | |
| Antibiotic target | ||
| Dose/route | ||
| Directed (blood culture based) antibiotic therapy | Yes/No | |
| Antibiotic type | ||
| Dose/route | ||
| Antibiotic therapy length (before CIED removal) | Days | |
| Antibiotic therapy length (after CIED removal) | Days | |
| Antibiotic therapy complications | Yes/No | |
| Type | ||
| Treatment | CIED removal | |
| Date of pulse generator removal | YYYY-MM-DD | |
| Date of leads removal | YYYY-MM-DD | |
| Lead extraction procedure | Simple traction, locking stylet, simple mechanical extraction sheath, rotational mechanical extraction sheath, laser sheath, femoral snare approach, internal jugular snare approach, other tools, concomitant percutaneous aspiration of vegetations, surgical extraction without CPB, surgical extraction with CPB | |
| Lead extraction outcomes | Complete/incomplete, major/minor complications | |
| Generator pocket tissue culture | Yes/No | |
| Main findings | ||
| Lead-tip culture | Yes/No | |
| Main findings | ||
| Wound closure | Primary wound closure—drain, delayed closure—negative pressure wound therapy | |
| Treatment | CIED reimplant | |
| Date of re-implantation | YYYY-MM-DD | |
| Type of CIED | Pacemaker, ICD, CRT | |
| Pulse generator pocket location | Pectoral/abdominal | |
| Lead access | Transvenous/epicardial/hybrid/leadless pacemaker/S-ICD | |
| Outcomes | ||
| Date of hospital discharge | YYYY-MM-DD | |
| Clinical condition at discharge | Resolved infection/unresolved infection/death | |
| Need of rehospitalization | Yes/No | |
| Date of rehospitalization | YYYY-MM-DD | |
| Recurrent infection | Yes/No | |
| Death | Yes/No | |
| Date of death | YYYY-MM-DD | |
| Cause of death | Lead extraction complications | |
| CIED infection (sepsis) | ||
| CIED dysfunction | ||
| Refractory heart failure | ||
| Cardiac death—other | ||
| Non-cardiac causes | ||
| Non-detected cause | ||
| Other | ||
CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; PM, pacemaker.
Gaps of evidence
The long-term mortality in patients with CIED infection is up to 2.5 times the mortality rate of non-infected patients. It is unclear whether it is related to the CIED infection itself, poor prognostic patient-related factors or inadequate management.
The optimal time for re-intervention (e.g. for lead repositioning) in order to reduce risk for infection is unclear.
The protective role of adhesive antibiotic surgical or other drapes for preventing infections is unclear.
An optimal risk stratification strategy or risk calculator to minimize risk for CIED infection is lacking.
The role of [18F]FDG PET/CT and scintigraphy with labelled leucocytes in the diagnosis and follow-up of occult and manifest CIED infections is of growing evidence of clinical utility but needs clarification.
Duration of postoperative antibiotic therapy following complete CIED system removal for infectious reasons is unclear.
The most optimal salvage strategy in severely ill or frail patients with CIED infections considered too high risk for complete system removal is unknown.
The appropriate timing of re-implantation after CIED infections is unclear.
In PM-dependent patients with CIED infection, the best strategy for permanent pacemaker treatment following lead extraction is unclear; an active-fixation lead ipsilaterally connected to an externalized PM followed by contralateral device implantation, an epicardial lead placement, or a LPM.
In case of a pocket infection with contralateral abandoned lead without systemic infection, it is unclear whether the contralateral abandoned lead should be extracted or not.
Summary of emerging messages and call for scientific evidence
Cardiac implantable electronic device infection rates seem lower (1.2%) in prospective studies6–10 than in retrospective registries (3.4%),11,12 which may be related to strengthened adherence to broad infection-preventative behaviours when participating in prospective studies. An assessment of the true infection rates in real-world practice is urgently warranted to more precisely be able to define the benefit of various structured preventive programmes, targeting modifiable risk factors, and developing risk stratification schemes for device implantation and re-implantations.
Although several guidelines and recommendations have been published from various medical societies for managing CIED infections, the EHRA worldwide survey on the clinical practice in managing CIED infections1 disclosed significant regional differences in current practice, incomplete adherence to guideline recommendations and a lack of profound knowledge in CIED infection management, which underline the need for more widespread and user-friendly international guidelines and implementation programs.
The timing of an extraction procedure should be without time delay after diagnosis of CIED infection since if performed within 3 days after hospitalization it results in significantly lower in-hospital mortality and shorter hospitalizations. Better diagnostic tools are warranted.
The most important procedure-related risk factors for device-related infections are pocket haematoma, long procedure duration, and re-intervention for lead repositioning. Strategies to prevent these risks should be better defined and undertaken more vigorously.
Antibacterial envelope reduces CIED infections in patients with risk factors for device-related infections and is recommended in high-risk patients. Further analysis derived from ‘real world’ practice will provide more information on its effectiveness and performance in less-selected settings. A risk stratification scheme is warranted to optimize its use.
Postoperative antibiotic is generally not recommended.
Needle aspiration and surgical debridement in cases of pocket infection as an attempt to avoid lead extraction is discouraged. Better diagnostic tools are warranted.
[18F]FDG PET/CT scanning or radiolabelled WBC scintigraphy or contrast-enhanced CT are recommended if suspected CIED-related infective endocarditis, positive blood cultures, and negative echocardiography.
[18F]FDG PET/CT scanning should be performed in case of S. aureus bacteraemia in the presence of CIED.
Prudent large national or international non-voluntary user-friendly quality registries of device implantations and its complications with regular monitoring and national requirements of minimal annual operators/centre device volumes may be the ultimate approach to reduce device-related infections.
Acknowledgements
The authors are thanks to EHRA Scientific Document Group: Dr. Nikolaos Dagres, Dr. Serge Boveda, Dr. Kevin Vernooy, Prof. Zbigniew Kalarus, Prof. Gulmira Kudaiberdieva, Dr. Georges H Mairesse, Prof. Valentina Kutyifa, Prof. Thomas Deneke, Pof. Jesper Hastrup Svendsen, Dr. Vassil B Traykov, Prof. Arthur Wilde, Prof. Frank R. Heinzel. The European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose and treat Cardiac Implantable Electronic Device infections was accepted in 2017 when Prof. Gregory YH Lip was chair of the EHRA Scientific Committee.
Contributor Information
ESC Scientific Document Group:
Zbigniew Kalarus, Serge Boveda, Nikolaos Dagres, Christopher A Rinaldi, Mauro Biffi, LászlóA Gellér, Adam Sokal, Ulrika Birgersdotter-Green, Nigel Lever, Mateusz Tajstra, Andrzej Kutarski, Diego A Rodríguez, Barbara Hasse, Annelies Zinkernagel, and EmanueleDurante Mangoni
Document reviewers
Zbigniew Kalarus (EHRA review coordinator), SMDZ in Zabrze, Medical University of Silesia, Katowice, Poland, Department of Cardiology, Silesian Center for Heart Diseases, Zabrze; Serge Boveda, Heart Rhythm Department, Clinique Pasteur Toulouse France; Nikolaos Dagres, Department of Electrophysiology, Heart Center Leipzig, Germany; Christopher A Rinaldi, Guy's and st Thomas' Hospitals London UK; Mauro Biffi, Cardio-Thoracic and Vascular Department, Azienda Ospedaliero-Universitaria di Bologna Italy; László A Gellér, Semmelweis University Heart and Vascular Center, Budapest, Hungary; Adam Sokal, Silesian Center of Heart Diseases, Ist Dept of Cardiology, Zabrze, Poland; Ulrika Birgersdotter-Green (Heart Rhythm Society, HRS), University Of California, San Diego, Cardiac Electrophysiology, 9444 Medical Center Dr, MC 7411, 92037 La Jolla, California, United States of America; Nigel Lever (Asia Pacific Heart Rhythm Society, APHRS), Green Lane Cardiovascular Services, Auckland City Hospital and University of Auckland, New Zealand; Mateusz Tajstra (European Association for Cardio-Thoracic Surgery, EACTS), 3rd Chair and Department of Cardiology, SMDZ in Zabrze, Silesian Center for Heart Diseases, Medical University of Silesia, Katowice, Poland; Andrzej Kutarski, Department of Cardiology Medical University of Lublin, Poland; Diego A. Rodríguez (Latin America Heart Rhythm Society LAHRS): Fundacion Cardioinfantil, Electrofisiologia, Cr 13B # 161 – 85, Centro de Especialistas, Torre I, CN 1010, 110131 Bogota, Colombia; Barbara Hasse (European Society of Clinical Microbiology and Infectious Diseases, ESCMID): University hospital Zurich, Division of Infectious Diseases and Hospital Epidemiology, Raemistrasse 100, 8091 Zurich, Switzerland; Annelies Zinkernagel (European Society of Clinical Microbiology and Infectious Diseases, ESCMID), Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Rämistr 100,8091 Zurich, Switzerland; Emanuele Durante Mangoni (International Society for Cardiovascular Infectious Diseases (ISCVID)). University of Campania ‘L. Vanvitelli’, Monaldi Hospital, Naples, Italy.
Conflict of interest:
Writing Group members
C Blomstrom-Lundqvist declares direct personal payment from Bayer, Sanofi Aventis, MSD, Medtronic and Boston. M. Bongiorni declares direct personal payment from Boston Scientific; G. Boriani direct personal payment from Boston Scientific, Medtronic and Biotronik; H. Burri from Biotronik and Medtronic; R. Costa from Medtronic and Biotronik; JC. Deharo from Boeringer-Ingelheim, Bayer Healthcare, Bristol Myers-Squibb, Biotronik, Abbott, Boston Scientific, Medtronic and Sorin Group and also departmental or institutional research funding from Abbott, Boston Scientific, Sorin Group and Biotronik. L. Epstein declares receiving direct personal payment from Abbott, Medtronic and Spectranetics; P. Erba from GE Healthcare, Gammaservizi and Sigma Tau and also royalties for intellectual property from Springer as well as departmental or institutional research funding from Sigma Tau and Gammaservizi, and is an ESMIT Board member and level-2 chair. JC. Nielsen declares receiving departmental or institutional research funding from Abbott and personal research funding from Novo Nordisk foundation. J.Poole declares receiving departmental or institutional research funding from Boston Scientifc, personal research funding from AtriCure and direct personal fees from Boston Scientific, Medtronic, EBR Systems, Kestra and Media Sphere. L. Saghy declares receiving personal fees from Johnson and Johnson and Medtronic. C. Starck declares receiving payment to his institution related to his activity as speaker fees, honoraria, consultancy, advisory board fees, investigator, committee member etc. from Angiodynamics, Medtronic, Spectranetics, Biotronik, Sorin and Cook Medical and departmental or institutional research funding from Cook Medical. C. Tascini declares receiving direct personal payment from Pfizer, Gilead, Astellas, Merck Sharp & Dohme, Angelini, Nordic Pharma and Biotest. V. Traykov declares receiving direct personal payment from Pfizer, Medtronic, Berlin Menarini, Bayer AG and Sandoz. The rest of the writing group have nothing to disclose.
Document reviewers
M. Biffi declares receiving direct personal payment from Boston Scientific, Biotronik and Medtronic. U Birgersdotter-Green declares receiving direct personal payment from Abbott, Medtronic, Biotronik. S. Boveda declares receiving direct personal payment from Medtronic, Microport, Boston Scientific and Zoll Medical. N. Dagres declares receiving institutional or departmental research funding under his direct responsibility from Abbott, Biotronik, Medtonic and Boston Scientific. EM Durante declares receiving institutional or departmental research funding under his direct responsibility from Pfizer and Merck Sharp & Dohme as well as direct personal payment from Pfizer, Merck Sharp & Dohme, Angelini, AbbiVie and Nordic Pharma. L. Geller declares receiving direct personal payment from Medtronic, Biotronik, Johnson and Johnson and Abbott Vascular. Z. Kalarus reports receiving direct personal fees from Abbott, Pfizer, Boehringer-Ingelheim, Bayer and Berlin Menarini. L. Nigel declares membership to Heart Rhythm New Zealand and does not report any financial conflicts. A. Rinaldi declares receiving direct personal payment from Phillips, Abbott and EBR Systems. He also declares payment to his department/institution or another body for his personal services: speaker fees, honoraria, consultancy, advisory board fees etc. from Abbott, Medtronic, Microport and Siemens Healthcare. Dr. Rinaldi also declared ownership of shares from HCA Diagnostic Centre. A. Rodriguez declares receiving direct personal payment from Biosense Webster and Medtronic as well as payment to his department/institution or another body for his personal services: speaker fees, honoraria, consultancy, advisory board fees etc. from Medtronic. A. Sokai declares receiving direct personal payment from Medtronic, Biotronik, Boston Scientific and BackBeat Medical. A. Zinkernagel declares holding a position of a Scientific officer of the Society of Clinical Microbiology and Infectious Diseases (ESCMID). The rest of the reviewers have nothing to disclose.
References
- 1. Traykov V, Bongiorni MG, Boriani G, Burri H, Costa R, Dagres N et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections; results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019;21:1270– 1279. [DOI] [PubMed] [Google Scholar]
- 2. Bongiorni MG, Kennergren C, Butter C, Deharo JC, Kutarski A, Rinaldi CA. et al. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J 2017;38:2995–3005. [DOI] [PubMed] [Google Scholar]
- 3. Johansen JB, Jorgensen OD, Moller M, Arnsbo P, Mortensen PT, Nielsen JC.. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011;32:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 5. Joy PS, Kumar G, Poole JE, London B, Olshansky B.. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm 2017;14:839–45. [DOI] [PubMed] [Google Scholar]
- 6. Ahsan SY, Saberwal B, Lambiase PD, Koo CY, Lee S, Gopalamurugan AB. et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace 2014;16:1482–9. [DOI] [PubMed] [Google Scholar]
- 7. Uslan DZ, Gleva MJ, Warren DK, Mela T, Chung MK, Gottipaty V. et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the replace registry. Pacing Clin Electrophysiol 2012;35:81–7. [DOI] [PubMed] [Google Scholar]
- 8. Biffi M, Ammendola E, Menardi E, Parisi Q, Narducci ML, De Filippo P. et al. Real-life outcome of implantable cardioverter-defibrillator and cardiac resynchronization defibrillator replacement/upgrade in a contemporary population: observations from the multicentre decode registry. Europace 2019;21:1527– 1536. [DOI] [PubMed] [Google Scholar]
- 9. Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran P. et al. Prevention of Arrhythmia Device Infection Trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–109. [DOI] [PubMed] [Google Scholar]
- 10. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E. et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 11. Clementy N, Carion PL, de Leotoing L, Lamarsalle L, Wilquin-Bequet F, Brown B. et al. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace 2018;20:1974–80. [DOI] [PubMed] [Google Scholar]
- 12. Ludwig S, Theis C, Brown B, Witthohn A, Lux W, Goette A.. Incidence and costs of cardiac device infections: retrospective analysis using German health claims data. J Comp Eff Res 2018;7:483–92. [DOI] [PubMed] [Google Scholar]
- 13. Da Costa A, Lelièvre H, Pha D, Kirkorian G, Célard M, Chevalier P. et al. Role of the preaxillary flora in pacemaker infections. Circulation 1998;97:1791–5. [DOI] [PubMed] [Google Scholar]
- 14. Uslan DZ, Sohail MR, St Sauver JL, Friedman PA, Hayes DL, Stoner SM. et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007;167:669–75. [DOI] [PubMed] [Google Scholar]
- 15. Darouiche RO. Device-associated infections: a macroproblem that starts with microadherence. Clin Infect Dis 2001;33:1567–72. [DOI] [PubMed] [Google Scholar]
- 16. Hussein AA, Baghdy Y, Wazni OM, Brunner MP, Kabbach G, Shao M. et al. Microbiology of cardiac implantable electronic device infections. JACC: Clin Electrophysiol 2016;2:498–505. [DOI] [PubMed] [Google Scholar]
- 17. Bongiorni MG, Tascini C, Tagliaferri E, Di Cori A, Soldati E, Leonildi A. et al. Microbiology of cardiac implantable electronic device infections. Europace 2012;14:1334–9. [DOI] [PubMed] [Google Scholar]
- 18. Wang R, Li X, Wang Q, Zhang Y, Wang H.. Microbiological characteristics and clinical features of cardiac implantable electronic device infections at a tertiary hospital in china. Front Microbiol 2017;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandoe JA, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P. et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325–59. [DOI] [PubMed] [Google Scholar]
- 20. Jan E, Camou F, Texier-Maugein J, Whinnett Z, Caubet O, Ploux S. et al. Microbiologic characteristics and in vitro susceptibility to antimicrobials in a large population of patients with cardiovascular implantable electronic device infection. J Cardiovasc Electrophysiol 2012;23:375–81. [DOI] [PubMed] [Google Scholar]
- 21. Polyzos KA, Konstantelias AA, Falagas ME.. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. [DOI] [PubMed] [Google Scholar]
- 22. Prutkin JM, Reynolds MR, Bao H, Curtis JP, Al-Khatib SM, Aggarwal S. et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the national cardiovascular data registry. Circulation 2014;130:1037–43. [DOI] [PubMed] [Google Scholar]
- 23. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB.. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J 2019;40:1862–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B. et al. ; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016;67:1300–08. [DOI] [PubMed] [Google Scholar]
- 25. Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH.. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002-2005. Circ Arrhythm Electrophysiol 2008;1:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mittal S, Shaw RE, Michel K, Palekar R, Arshad A, Musat D. et al. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014;11:595–601. [DOI] [PubMed] [Google Scholar]
- 27. Shariff N, Eby E, Adelstein E, Jain S, Shalaby A, Saba S. et al. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol 2015;26:783–9. [DOI] [PubMed] [Google Scholar]
- 28. Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N. et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007;116:1349–55. [DOI] [PubMed] [Google Scholar]
- 29. Birnie DH, Healey JS, Wells GA, Ayala-Paredes F, Coutu B, Sumner GL. et al. Continued vs. Interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J 2018;39:3973–79. [DOI] [PubMed] [Google Scholar]
- 30. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD. et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–93. [DOI] [PubMed] [Google Scholar]
- 31. Kutinsky IB, Jarandilla R, Jewett M, Haines DE.. Risk of hematoma complications after device implant in the clopidogrel era. Circ Arrhythm Electrophysiol 2010;3:312–18. [DOI] [PubMed] [Google Scholar]
- 32. Robinson M, Healey JS, Eikelboom J, Schulman SAM, Morillo CA, Nair GM. et al. Postoperative low-molecular-weight heparin bridging is associated with an increase in wound hematoma following surgery for pacemakers and implantable defibrillators. Pacing Clin Electrophysiol 2009;32:378–2. [DOI] [PubMed] [Google Scholar]
- 33. Du L, Zhang Y, Wang W, Hou Y.. Perioperative anticoagulation management in patients on chronic oral anticoagulant therapy undergoing cardiac devices implantation: a meta-analysis. Pacing Clin Electrophysiol 2014;37:1573–86. [DOI] [PubMed] [Google Scholar]
- 34. Haines DE, Beheiry S, Akar JG, Baker JL, Beinborn D, Beshai JF. et al. Heart rhythm society expert consensus statement on electrophysiology laboratory standards: process, protocols, equipment, personnel, and safety. Heart Rhythm 2014;11:e9–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanner J, Norrie P, Melen K.. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev 2011:CD004122. [DOI] [PubMed] [Google Scholar]
- 36. Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM. et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 2010;362:18–26. [DOI] [PubMed] [Google Scholar]
- 37. Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V. et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (clean): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet (Lond, Engl) 2015;386:2069–77. [DOI] [PubMed] [Google Scholar]
- 38. Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR. et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007;49:1851–59. [DOI] [PubMed] [Google Scholar]
- 39. Asif A, Carrillo R, Garisto J-D, Lopera G, Ladino M, Barakat U. et al. Epicardial cardiac rhythm devices for dialysis patients: minimizing the risk of infection and preserving central veins. Semin Dial 2012;25:88–94. [DOI] [PubMed] [Google Scholar]
- 40. El-Chami MF, Soejima K, Piccini JP, Reynolds D, Ritter P, Okabe T. et al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol 2019;24: doi:10.1111/pace.13752. [DOI] [PubMed] [Google Scholar]
- 41. El-Chami MF, Clementy N, Garweg C, Omar R, Duray GZ, Gornick CC. et al. Leadless pacemaker implantation in hemodialysis patients: experience with the Micra transcatheter pacemaker. JACC: Clin Electrophysiol 2019;5:162–70. [DOI] [PubMed] [Google Scholar]
- 42. Pokorney SD, Mi X, Lewis RK, Greiner M, Epstein LM, Carrillo RG. et al. Outcomes associated with extraction versus capping and abandoning pacing and defibrillator leads. Circulation 2017;136:1387–95. [DOI] [PubMed] [Google Scholar]
- 43. Hussein AA, Tarakji KG, Martin DO, Gadre A, Fraser T, Kim A. et al. Cardiac implantable electronic device infections: added complexity and suboptimal outcomes with previously abandoned leads. JACC: Clin Electrophysiol 2017;3:1–9. [DOI] [PubMed] [Google Scholar]
- 44. de Vries FE, Gans SL, Solomkin JS, Allegranzi B, Egger M, Dellinger EP. et al. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg 2017;104:e95–e105. [DOI] [PubMed] [Google Scholar]
- 45. Merino JL, Arribas F, Botto GL, Huikuri H, Kraemer LI, Linde C. et al. Core curriculum for the heart rhythm specialist. Europace 2009;11:1–26. [DOI] [PubMed] [Google Scholar]
- 46. Bode LGM, Kluytmans J, Wertheim HFL, Bogaers D, Vandenbroucke-Grauls C, Roosendaal R. et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- 47. Franco L, Cota GF, Pinto TS, Ercole FF.. Preoperative bathing of the surgical site with chlorhexidine for infection prevention: systematic review with meta-analysis. Am J Infect Control 2017;45:343–9. [DOI] [PubMed] [Google Scholar]
- 48. de Oliveira JC, Martinelli M, Nishioka SA, Varejão T, Uipe D, Pedrosa AA. et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythmia Electrophysiol 2009;2:29–34. [DOI] [PubMed] [Google Scholar]
- 49. Da Costa A, Kirkorian G, Cucherat M, Delahaye F, Chevalier P, Cerisier A. et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998;97:1796–801. [DOI] [PubMed] [Google Scholar]
- 50. Webster J, Alghamdi A.. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev 2015;4:CD006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suding P, Nguyen T, Gordon I, Wilson SE.. Glove powder increases Staphylococcus aureus abscess rate in a rat model. Surg Infect 2010;11:133–5. [DOI] [PubMed] [Google Scholar]
- 52.NICE Guideline Updates Team. Surgical Site Infections: Prevention and Treatment. Nice Guideline No. 125. London, UK: National Institute for Health and Care Excellence; 2019. [PubMed] [Google Scholar]
- 53. Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau W. et al. Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010;12:58–63. [DOI] [PubMed] [Google Scholar]
- 54. Lakkireddy D, Pillarisetti J, Atkins D, Biria M, Reddy M, Murray C. et al. Impact of pocket revision on the rate of infection and other complications in patients requiring pocket manipulation for generator replacement and/or lead replacement or revision (make it clean): a prospective randomized study. Heart Rhythm 2015;12:950–6. [DOI] [PubMed] [Google Scholar]
- 55. Grubb B, Welch M, Karabin B, Foster W, Zhang D, Kanjwal K.. Initial experience with a technique for wound closure after cardiac device implantation designed to reduce infection and minimize tissue scar formation. Am J Therap 2012;19:88–91. [DOI] [PubMed] [Google Scholar]
- 56. Klug D, Wallet F, Lacroix D, Marquie C, Kouakam C, Kacet S. et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart 2004;90:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy L, Group ESD et al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace 2018;20:1217–17. [DOI] [PubMed] [Google Scholar]
- 58. Knigina L, Kuhn C, Kutschka I, Oswald H, Klein G, Haverich A. et al. Treatment of patients with recurrent or persistent infection of cardiac implantable electronic devices. Europace 2010;12:1275–81. [DOI] [PubMed] [Google Scholar]
- 59. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F. et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 60. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T. et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- 61. Klug D, Lacroix D, Savoye C, Goullard L, Grandmougin D, Hennequin JL. et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation 1997;95:2098–107. [DOI] [PubMed] [Google Scholar]
- 62. Cacoub P, Leprince P, Nataf P, Hausfater P, Dorent R, Wechsler B. et al. Pacemaker infective endocarditis. Am J Cardiol 1998;82:480–4. [DOI] [PubMed] [Google Scholar]
- 63. Lennerz C, Vrazic H, Haller B, Braun S, Petzold T, Ott I. et al. Biomarker-based diagnosis of pacemaker and implantable cardioverter defibrillator pocket infections: a prospective, multicentre, case-control evaluation. PLoS One 2017;12:e0172384.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cornelissen CG, Frechen DA, Schreiner K, Marx N, Krüger S.. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis 2013;13:272.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB. et al. American Heart Association Rheumatic Fever Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular, Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458–77. [DOI] [PubMed] [Google Scholar]
- 66. Dy Chua J, Abdul-Karim A, Mawhorter S, Procop GW, Tchou P, Niebauer M.. The role of swab and tissue culture in the diagnosis of implantable cardiac device infection. Pacing Clin Electrophysiol 2005;28:1276–81. [DOI] [PubMed] [Google Scholar]
- 67. Forward KR. . An evaluation of extended incubation time with blind subculture of blood cultures in patients with suspected endocarditis. Can J Infect Dis Med Microbiol 2006;17:186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mason PK, Dimarco JP, Ferguson JD, Mahapatra S, Mangrum JM, Bilchick KC. et al. Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin Electrophysiol 2011;34:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Oliva A, Nguyen BL, Mascellino MT, D'Abramo A, Iannetta M, Ciccaglioni A. et al. Sonication of explanted cardiac implants improves microbial detection in cardiac device infections. J Clin Microbiol 2013;51:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Viola GM, Mansouri MD, Nasir N, Darouiche RO.. Incubation alone is adequate as a culturing technique for cardiac rhythm management devices. J Clin Microbiol 2009;47:4168–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrison JL, Prendergast BD, Sandoe JA.. Guidelines for the diagnosis, management and prevention of implantable cardiac electronic device infection. Heart 2015;101:250–2. [DOI] [PubMed] [Google Scholar]
- 72. Viola GM, Awan LL, Darouiche RO.. Nonstaphylococcal infections of cardiac implantable electronic devices. Circulation 2010;121:2085–91. [DOI] [PubMed] [Google Scholar]
- 73. Nagpal A, Baddour LM, Sohail MR.. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ Arrhythm Electrophysiol 2012;5:433–41. [DOI] [PubMed] [Google Scholar]
- 74. Vilacosta I, Sarriá C, San Román JA, Jiménez J, Castillo JA, Iturralde E. et al. Usefulness of transesophageal echocardiography for diagnosis of infected transvenous permanent pacemakers. Circulation 1994;89:2684–87. [DOI] [PubMed] [Google Scholar]
- 75. Golzio PG, Errigo D, Peyracchia M, Gallo E, Frea S, Castagno D. et al. Prevalence and prognosis of lead masses in patients with cardiac implantable electronic devices without infection. J Cardiovasc Med 2019;20:372–8. [DOI] [PubMed] [Google Scholar]
- 76. Downey BC, Juselius WE, Pandian NG, Estes NA 3rd, Link MS.. Incidence and significance of pacemaker and implantable cardioverter-defibrillator lead masses discovered during transesophageal echocardiography. Pacing Clin Electrophysiol 2011;34:679–83. [DOI] [PubMed] [Google Scholar]
- 77. Bongiorni MG, Di Cori A, Soldati E, Zucchelli G, Arena G, Segreti L. et al. Intracardiac echocardiography in patients with pacing and defibrillating leads: a feasibility study. Echocardiography 2008;25:632–8. [DOI] [PubMed] [Google Scholar]
- 78. Narducci ML, Pelargonio G, Russo E, Marinaccio L, Di Monaco A, Perna F. et al. Usefulness of intracardiac echocardiography for the diagnosis of cardiovascular implantable electronic device-related endocarditis. J Am Coll Cardiol 2013;61:1398–1405. [DOI] [PubMed] [Google Scholar]
- 79. Chang D, Gabriels J, Laighold S, Williamson AK, Ismail H, Epstein LM.. A novel diagnostic approach to a mass on a device lead. HeartRhythm Case Rep 2019;5:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Diemberger I, Biffi M, Lorenzetti S, Martignani C, Raffaelli E, Ziacchi M. et al. Predictors of long-term survival free from relapses after extraction of infected CIED. Europace 2018;20:1018–27. [DOI] [PubMed] [Google Scholar]
- 81. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R. et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 82. Incani A, Hair C, Purnell P, O’Brien DP, Cheng AC, Appelbe A. et al. Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013;32:1003–8. [DOI] [PubMed] [Google Scholar]
- 83. Rasmussen RV, Høst U, Arpi M, Hassager C, Johansen HK, Korup E. et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011;12:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferreira-González I, Gonzàlez-Alujas MT. et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation 2015;132:1113–26. [DOI] [PubMed] [Google Scholar]
- 85. Erba PA, Lancellotti P, Vilacosta I, Gaemperli O, Rouzet F, Hacker M. et al. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur J Nucl Med Mol Imaging 2018;45:1795–815. [DOI] [PubMed] [Google Scholar]
- 86. Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EMM, Oyen W.. Cost-effectiveness of routine 18F-FDG PET/CT in high-risk patients with Gram-positive bacteremia. J Nucl Med 2011;52:1673–8. [DOI] [PubMed] [Google Scholar]
- 87. Berrevoets MAH, Kouijzer IJE, Aarntzen E, Janssen MJR, De Geus-Oei L-F, Wertheim HFL. et al. 18F-FDG PET/CT optimizes treatment in Staphylococcus aureus bacteremia and is associated with reduced mortality. J Nucl Med 2017;58:1504–10. [DOI] [PubMed] [Google Scholar]
- 88. Amraoui S, Tlili G, Sohal M, Berte B, Hindié E, Ritter P. et al. Contribution of pet imaging to the diagnosis of septic embolism in patients with pacing lead endocarditis. JACC Cardiovasc Imaging 2016;9:283–90. [DOI] [PubMed] [Google Scholar]
- 89. Erba PA, Sollini M, Conti U, Bandera F, Tascini C, De Tommasi SM. et al. Radiolabeled wbc scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc Imaging 2013;6:1075–86. [DOI] [PubMed] [Google Scholar]
- 90. Sollini M, Berchiolli R, Delgado Bolton RC, Rossi A, Kirienko M, Boni R. et al. The “3m” approach to cardiovascular infections: multimodality, multitracers, and multidisciplinary. Semin Nucl Med 2018;48:199–224. [DOI] [PubMed] [Google Scholar]
- 91. Paparoupa M, Spineli L, Framke T, Ho H, Schuppert F, Gillissen A.. Pulmonary embolism in pneumonia: still a diagnostic challenge? Results of a case-control study in 100 patients. Dis Markers 2016;2016:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Narducci ML, Di Monaco A, Pelargonio G, Leoncini E, Boccia S, Mollo R. et al. Presence of ‘ghosts’ and mortality after transvenous lead extraction. Europace 2017;19:432–40. [DOI] [PubMed] [Google Scholar]
- 93. Park S-J, Gentry JL, Varma N, Wazni O, Tarakji KG, Mehta A. et al. Transvenous extraction of pacemaker and defibrillator leads and the risk of tricuspid valve regurgitation. JACC: Clin Electrophysiol 2018;4:1421–8. [DOI] [PubMed] [Google Scholar]
- 94. Pijl JP, Glaudemans A, Slart R, Yakar D, Wouthuyzen-Bakker M, Kwee TC.. FDG-PET/CT for detecting an infection focus in patients with bloodstream infection: factors affecting diagnostic yield. Clin Nucl Med 2019;44:99–106. [DOI] [PubMed] [Google Scholar]
- 95. Berrevoets MAH, Kouijzer IJE, Slieker K, Aarntzen E, Kullberg BJ, Oever JT. et al. 18F-FDG PET/CT-guided treatment duration in patients with high-risk Staphylococcus aureus bacteremia: a proof of principle. J Nucl Med 2019;60:998–1002. [DOI] [PubMed] [Google Scholar]
- 96. Mandry D, Tatopoulos A, Chevalier-Mathias E, Lemarié J, Bollaert P-E, Roch V. et al. 18F-fluorodeoxyglucose positron emission tomography combined with whole-body computed tomographic angiography in critically ill patients with suspected severe sepsis with no definite diagnosis. Eur J Nucl Med Mol Imaging 2014;41:1924–30. [DOI] [PubMed] [Google Scholar]
- 97. Vos FJ, Kullberg BJ, Sturm PD, Krabbe PFM, van Dijk APJ, Wanten GJ. et al. Metastatic infectious disease and clinical outcome in Staphylococcus aureus and Streptococcus species bacteremia. Medicine 2012;91:86–94. [DOI] [PubMed] [Google Scholar]
- 98. Botelho-Nevers E, Thuny F, Casalta JP, Richet H, Gouriet F, Collart F. et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009;169:1290–8. [DOI] [PubMed] [Google Scholar]
- 99. Juneau D, Golfam M, Hazra S, Zuckier LS, Garas S, Redpath C. et al. Positron emission tomography and single-photon emission computed tomography imaging in the diagnosis of cardiac implantable electronic device infection: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2017;10:e005772.. [DOI] [PubMed] [Google Scholar]
- 100. Mahmood M, Kendi AT, Farid S, Ajmal S, Johnson GB, Baddour LM. et al. Role of 18F-FDG PET/CT in the diagnosis of cardiovascular implantable electronic device infections: a meta-analysis. J Nucl Cardiol 2019;26:958–70. [DOI] [PubMed] [Google Scholar]
- 101. Diemberger I, Bonfiglioli R, Martignani C, Graziosi M, Biffi M, Lorenzetti S. et al. Contribution of pet imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: a prospective single center study. Eur J Nucl Med Mol Imaging 2019;46:194–205. [DOI] [PubMed] [Google Scholar]
- 102. Lebeaux D, Fernández-Hidalgo N, Chauhan A, Lee S, Ghigo J-M, Almirante B. et al. Management of infections related to totally implantable venous-access ports: challenges and perspectives. Lancet Infect Dis 2014;14:146–59. [DOI] [PubMed] [Google Scholar]
- 103. Peacock JE, Stafford JM, Le K, Sohail MR, Baddour LM, Prutkin JM. et al. Attempted salvage of infected cardiovascular implantable electronic devices: are there clinical factors that predict success? Pacing Clin Electrophysiol 2018;41:524–31. [DOI] [PubMed] [Google Scholar]
- 104. Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL. et al. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm 2011;8:1678–85. [DOI] [PubMed] [Google Scholar]
- 105. Patel D, Khan F, Shah H, Bhattacharya S, Adelstein E, Saba S.. Cardiac implantable electronic device lead extraction in patients with underlying infection using open thoracotomy or percutaneous techniques. Cardiol J 2015;22:68–74. [DOI] [PubMed] [Google Scholar]
- 106. Schaerf RHM, Najibi S, Conrad J.. Percutaneous vacuum-assisted thrombectomy device used for removal of large vegetations on infected pacemaker and defibrillator leads as an adjunct to lead extraction. J Atr Fibrillation 2016;9:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Starck CT, Eulert-Grehn J, Kukucka M, Eggert-Doktor D, Dreizler T, Haupt B. et al. Managing large lead vegetations in transvenous lead extractions using a percutaneous aspiration technique. Expert Rev Med Devices 2018;15:757–61. [DOI] [PubMed] [Google Scholar]
- 108. Lakshmanadoss U, Nuanez B, Kutinsky I, Khalid R, Haines DE, Wong WS.. Incidence of pocket infection postcardiac device implantation using antibiotic versus saline solution for pocket irrigation. Pacing Clin Electrophysiol 2016;39:978–84. [DOI] [PubMed] [Google Scholar]
- 109. Le KY, Sohail MR, Friedman PA, Uslan DZ, Cha SS, Hayes DL. et al. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol 2012;110:1143–9. [DOI] [PubMed] [Google Scholar]
- 110. Madhavan M, Sohail MR, Friedman PA, Hayes DL, Steckelberg JM, Wilson WR, null n et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia caused by Gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol 2010;3:639–45. [DOI] [PubMed] [Google Scholar]
- 111. Maskarinec SA, Thaden JT, Cyr DD, Ruffin F, Souli M, Fowler VG.. The risk of cardiac device-related infection in bacteremic patients is species specific: results of a 12-year prospective cohort. Open Forum Infect Dis 2017;4:ofx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Uslan DZ, Sohail MR, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM. et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with Gram-negative bacteremia. Clin Infect Dis 2006;43:731–6. [DOI] [PubMed] [Google Scholar]
- 113. Huang X-M, Fu H-X, Zhong L, Cao J, Asirvatham SJ, Baddour LM. et al. Outcomes of transvenous lead extraction for cardiovascular implantable electronic device infections in patients with prosthetic heart valves. Circulation 2016;9:e004188.. [DOI] [PubMed] [Google Scholar]
- 114. Viganego F, O'Donoghue S, Eldadah Z, Shah MH, Rastogi M, Mazel JA. et al. Effect of early diagnosis and treatment with percutaneous lead extraction on survival in patients with cardiac device infections. Am J Cardiol 2012;109:1466–71. [DOI] [PubMed] [Google Scholar]
- 115. Rusanov A, Spotnitz HM.. A 15-year experience with permanent pacemaker and defibrillator lead and patch extractions. Ann Thorac Surg 2010;89:44–50. [DOI] [PubMed] [Google Scholar]
- 116. Meier-Ewert HK, Gray M-E, John RM.. Endocardial pacemaker or defibrillator leads with infected vegetations: a single-center experience and consequences of transvenous extraction. Am Heart J 2003;146:339–44. [DOI] [PubMed] [Google Scholar]
- 117. Pérez Baztarrica G, Gariglio L, Salvaggio F, Reolón E, Blanco N, Mazzetti H. et al. Transvenous extraction of pacemaker leads in infective endocarditis with vegetations ≥20 mm: our experience. Clin Cardiol 2012;35:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tan EM, DeSimone DC, Sohail MR, Baddour LM, Wilson WR, Steckelberg JM. et al. Outcomes in patients with cardiovascular implantable electronic device infection managed with chronic antibiotic suppression. Clin Infect Dis 2017;64:1516–21. [DOI] [PubMed] [Google Scholar]
- 119. Sohail MR, Palraj BR, Khalid S, Uslan DZ, Al-Saffar F, Friedman PA. et al. Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (PREDICT-SAB). Circ Arrhythm Electrophysiol 2015;8:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J, UKCIRG et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (arrest): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet (Lond, Engl) 2018;391:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bongiorni MG, Marinskis G, Lip GY, Svendsen JH, Dobreanu D, Blomström-Lundqvist C. Scientific Initiative Committee; European Heart Rhythm Association. How European centres diagnose, treat, and prevent CIED infections: results of an European Heart Rhythm Association survey. Europace 2012;14:1666–9. [DOI] [PubMed] [Google Scholar]
- 122. Grammes JA, Schulze CM, Al-Bataineh M, Yesenosky GA, Saari CS, Vrabel MJ. et al. Percutaneous pacemaker and implantable cardioverter-defibrillator lead extraction in 100 patients with intracardiac vegetations defined by transesophageal echocardiogram. J Am Coll Cardiol 2010;55:886–94. [DOI] [PubMed] [Google Scholar]
- 123. Tarakji KG, Chan EJ, Cantillon DJ, Doonan AL, Hu T, Schmitt S. et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010;7:1043–7. [DOI] [PubMed] [Google Scholar]
- 124. Braun MU, Rauwolf T, Bock M, Kappert UTZ, Boscheri A, Schnabel A. et al. Percutaneous lead implantation connected to an external device in stimulation-dependent patients with systemic infection—a prospective and controlled study. Pacing Clin Electrophysiol 2006;29:875–9. [DOI] [PubMed] [Google Scholar]
- 125. Kornberger A, Schmid E, Kalender G, Stock UA, Doernberger V, Khalil M. et al. Bridge to recovery or permanent system implantation: an eight-year single-center experience in transvenous semipermanent pacing. Pacing Clin Electrophysiol 2013;36:1096–103. [DOI] [PubMed] [Google Scholar]
- 126. Kawata H, Pretorius V, Phan H, Mulpuru S, Gadiyaram V, Patel J. et al. Utility and safety of temporary pacing using active fixation leads and externalized re-usable permanent pacemakers after lead extraction. Europace 2013;15:1287–91. [DOI] [PubMed] [Google Scholar]
- 127. Pecha S, Aydin MA, Yildirim Y, Sill B, Reiter B, Wilke I. et al. Transcutaneous lead implantation connected to an externalized pacemaker in patients with implantable cardiac defibrillator/pacemaker infection and pacemaker dependency. Europace 2013;15:1205–9. [DOI] [PubMed] [Google Scholar]
- 128. Zucchelli G, Bongiorni MG, Di Cori A, Soldati E, Solarino G, Fabiani I. et al. Cardiac resynchronization therapy after coronary sinus lead extraction: feasibility and mid-term outcome of transvenous reimplantation in a tertiary referral centre. Europace 2012;14:515–21. [DOI] [PubMed] [Google Scholar]
- 129. Bongiorni MG, Della Tommasina V, Barletta V, Di Cori A, Rogani S, Viani S. et al. Feasibility and long-term effectiveness of a non-apical Micra pacemaker implantation in a referral centre for lead extraction. Europace 2019;21:114–20. [DOI] [PubMed] [Google Scholar]
- 130. Beurskens NEG, Tjong FVY, Dasselaar KJ, Kuijt WJ, Wilde AAM, Knops RE.. Leadless pacemaker implantation after explantation of infected conventional pacemaker systems: a viable solution? Heart Rhythm 2019;16:66–71. [DOI] [PubMed] [Google Scholar]
- 131. Kypta A, Blessberger H, Kammler J, Lambert T, Lichtenauer M, Brandstaetter W. et al. Leadless cardiac pacemaker implantation after lead extraction in patients with severe device infection. J Cardiovasc Electrophysiol 2016;27:1067–71. [DOI] [PubMed] [Google Scholar]
- 132. Viani S, Migliore F, Tola G, Pisanò ECL, Russo AD, Luzzi G. et al. Use and outcomes of subcutaneous implantable cardioverter-defibrillator (ICD) after transvenous ICD extraction: an analysis of current clinical practice and a comparison with transvenous ICD reimplantation. Heart Rhythm 2019;16:564–71. [DOI] [PubMed] [Google Scholar]
- 133. Boersma L, Burke MC, Neuzil P, Lambiase P, Friehling T, Theuns DA. et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous ICD extraction. Heart Rhythm 2016;13:157–64. [DOI] [PubMed] [Google Scholar]
- 134. Darouiche R, Mosier M, Voigt J.. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. Pacing Clin Electrophysiol 2012;35:1348–60. [DOI] [PubMed] [Google Scholar]
- 135. Deharo JC, Bongiorni MG, Rozkovec A, Bracke F, Defaye P, Fernandez-Lozano I. et al. European Heart Rhythm Association. Pathways for training and accreditation for transvenous lead extraction: a European Heart Rhythm Association position paper. Europace 2012;14:124–34. [DOI] [PubMed] [Google Scholar]
- 136. El-Chami MF, Johansen JB, Zaidi A, Faerestrand S, Reynolds D, Garcia-Seara J. et al. Leadless pacemaker implant in patients with pre-existing infections: results from the Micra postapproval registry. J Cardiovasc Electrophysiol 2019;30:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S. et al. ; Micra Transcatheter Pacing Study Group. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 138. Brouwer TF, Yilmaz D, Lindeboom R, Buiten MS, Olde Nordkamp LRA, Schalij MJ. et al. Long-term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J Am Coll Cardiol 2016;68:2047–55. [DOI] [PubMed] [Google Scholar]
- 139. Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P. et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the effortless study. J Am Coll Cardiol 2017;70:830–41. [DOI] [PubMed] [Google Scholar]
- 140. Rizwan Sohail M, Henrikson CA, Jo Braid-Forbes M, Forbes KF, Lerner DJ.. Increased long-term mortality in patients with cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015;38:231–9. [DOI] [PubMed] [Google Scholar]
- 141. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ.. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011;171:1821–8. [DOI] [PubMed] [Google Scholar]
- 142. Greenspon AJ, Eby EL, Petrilla AA, Sohail MR.. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol 2018;41:495–503. [DOI] [PubMed] [Google Scholar]
- 143. Maytin M, Jones SO, Epstein LM.. Long-term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol 2012;5:252–7. [DOI] [PubMed] [Google Scholar]
- 144. Lee DH, Gracely EJ, Aleem SY, Kutalek SP, Vielemeyer O.. Differences of mortality rates between pocket and nonpocket cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol 2015;38:1456–63. [DOI] [PubMed] [Google Scholar]
- 145. Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL.. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014;16:1490–5. [DOI] [PubMed] [Google Scholar]
- 146. Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ.. Comparison of mortality in women versus men with infections involving cardiovascular implantable electronic device. Am J Cardiol 2013;112:1403–9. [DOI] [PubMed] [Google Scholar]
- 147. Guha A, Maddox WR, Colombo R, Nahman NS Jr, Kintziger KW, Waller JL. et al. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm 2015;12:2395–401. [DOI] [PubMed] [Google Scholar]
- 148. Deharo JC, Quatre A, Mancini J, Khairy P, Le Dolley Y, Casalta JP. et al. Long-term outcomes following infection of cardiac implantable electronic devices: a prospective matched cohort study. Heart 2012;98:724–31. [DOI] [PubMed] [Google Scholar]
- 149. Rickard J, Tarakji K, Cheng A, Spragg D, Cantillon DJ, Martin DO. et al. Survival of patients with biventricular devices after device infection, extraction, and reimplantation. JACC Heart Fail 2013;1:508–13. [DOI] [PubMed] [Google Scholar]
- 150. Armaganijan LV, Toff WD, Nielsen JC, Andersen HR, Connolly SJ, Ellenbogen KA. et al. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol 2012;35:131–4. [DOI] [PubMed] [Google Scholar]
- 151. Habib A, Le KY, Baddour LM, Friedman PA, Hayes DL, Lohse CM. et al. Mayo Cardiovascular Infections Study Group. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol 2013;111:874–9. [DOI] [PubMed] [Google Scholar]
- 152. Nielsen JC, Gerdes JC, Varma N.. Infected cardiac-implantable electronic devices: prevention, diagnosis, and treatment. Eur Heart J 2015;36:2484–90. [DOI] [PubMed] [Google Scholar]
- 153. Voigt A, Shalaby A, Saba S.. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–19. [DOI] [PubMed] [Google Scholar]
- 154. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA. et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol 2014;63:747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Green AR, Leff B, Wang Y, Spatz ES, Masoudi FA, Peterson PN. et al. Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes 2016;9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kramer DB, Tsai T, Natarajan P, Tewksbury E, Mitchell SL, Travison TG.. Frailty, physical activity, and mobility in patients with cardiac implantable electrical devices. J Am Heart Assoc 2017;6:e004659.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Farage MA, Miller KW, Elsner P, Maibach HI.. Structural characteristics of the aging skin: a review. Cutan Ocul Toxicol 2007;26:343–57. [DOI] [PubMed] [Google Scholar]
- 158. Poole J, Larson L, Olshansky B.. Cardiac Implantable Electronic Device Surgical Implant. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 159. Atallah J, Erickson CC, Cecchin F, Dubin AM, Law IH, Cohen MI. et al. Multi-institutional study of implantable defibrillator lead performance in children and young adults: results of the Pediatric Lead Extractability and Survival Evaluation (PLEASE) study. Circulation 2013;127:2393–402. [DOI] [PubMed] [Google Scholar]
- 160. Padfield GJ, Steinberg C, Bennett MT, Chakrabarti S, Deyell MW, Bashir J. et al. Preventing cardiac implantable electronic device infections. Heart Rhythm 2015;12:2344–56. [DOI] [PubMed] [Google Scholar]
- 161. Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R. et al. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 2010;121:1691–7. [DOI] [PubMed] [Google Scholar]
- 162. Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R. et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the replace registry. Circulation 2010;122:1553–61. [DOI] [PubMed] [Google Scholar]
- 163. Fortescue EB, Berul CI, Cecchin F, Walsh EP, Triedman JK, Alexander ME.. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm 2004;1:150–9. [DOI] [PubMed] [Google Scholar]
- 164. Gleva MJ, Wang Y, Curtis JP, Berul CI, Huddleston CB, Poole JE.. Complications associated with implantable cardioverter defibrillators in adults with congenital heart disease or left ventricular noncompaction cardiomyopathy (from the NCDR® implantable cardioverter-defibrillator registry). Am J Cardiol 2017;120:1891–8. [DOI] [PubMed] [Google Scholar]
- 165. Berul CI, Van Hare GF, Kertesz NJ, Dubin AM, Cecchin F, Collins KK. et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol 2008;51:1685–91. [DOI] [PubMed] [Google Scholar]
- 166. Vehmeijer JT, Brouwer TF, Limpens J, Knops RE, Bouma BJ, Mulder BJ. et al. Implantable cardioverter-defibrillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J 2016;37:1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cecchin F, Atallah J, Walsh EP, Triedman JK, Alexander ME, Berul CI.. Lead extraction in pediatric and congenital heart disease patients. Circ Arrhythm Electrophysiol 2010;3:437–44. [DOI] [PubMed] [Google Scholar]
- 168. Cannon BC, Friedman RA, Fenrich AL, Fraser CD, McKenzie ED, Kertesz NJ.. Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. Pacing Clin Electrophysiol 2006;29:181–7. [DOI] [PubMed] [Google Scholar]
- 169. Konta L, Chubb MH, Bostock J, Rogers J, Rosenthal E.. Twenty-seven years experience with transvenous pacemaker implantation in children weighing <10 kg. Circ Arrhythm Electrophysiol 2016;9:e003422. [DOI] [PubMed] [Google Scholar]
- 170. Molina JE. Surgical options for endocardial lead placement when upper veins are obstructed or nonusable. J Interv Card Electrophysiol 2004;11:149–54. [DOI] [PubMed] [Google Scholar]
- 171. Silvetti MS, Drago F.. Upgrade of single chamber pacemakers with transvenous leads to dual chamber pacemakers in pediatric and young adult patients. Pacing Clin Electrophysiol 2004;27:1094–8. [DOI] [PubMed] [Google Scholar]
- 172. Suzuki S, Motohashi S, Matsumoto M.. Surgical techniques for implanting implantable cardioverter defibrillators in children and infants. Surg Today 2014;44:1801–6. [DOI] [PubMed] [Google Scholar]
- 173. McCanta AC, Tanel RE, Gralla J, Runciman DM, Collins KK.. The fate of nontargeted endocardial leads during the extraction of one or more targeted leads in pediatrics and congenital heart disease. Pacing and Clinical Electrophysiology 2014;37:104–8. [DOI] [PubMed] [Google Scholar]
- 174. Gillette PC, Edgerton J, Kratz J, Zeigler V.. The subpectoral pocket: the preferred implant site for pediatric pacemakers. Pacing Clin Electrophysiol 1991;14:1089–92. [DOI] [PubMed] [Google Scholar]
- 175. D'Souza BA, Epstein AE, Garcia FC, Kim YY, Agarwal SC, Belott PH. et al. Outcomes in patients with congenital heart disease receiving the subcutaneous implantable-cardioverter defibrillator: results from a pooled analysis from the IDE study and the EFFORTLESS S-ICD registry. JACC Clin Electrophysiol 2016;2:615–22. [DOI] [PubMed] [Google Scholar]
- 176. Moore JP, Mondesert B, Lloyd MS, Cook SC, Zaidi AN, Pass RH. et al. Alliance for Adult Research in Congenital Cardiology. Clinical experience with the subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. Circ Arrhythm Electrophysiol 2016;9:e004338.. [DOI] [PubMed] [Google Scholar]
- 177. Bordachar P, Marquie C, Pospiech T, Pasquie JL, Jalal Z, Haissaguerre M. et al. Subcutaneous implantable cardioverter defibrillators in children, young adults and patients with congenital heart disease. Int J Cardiol 2016;203:251–8. [DOI] [PubMed] [Google Scholar]
- 178. Celiker A, Olgun H, Karagoz T, Ozer S, Ozkutlu S, Alehan D.. Midterm experience with implantable cardioverter-defibrillators in children and young adults. Europace 2010;12:1732–8. [DOI] [PubMed] [Google Scholar]
- 179. Ferrero P, Ali H, Barman P, Foresti S, Lupo P, D’Elia E. et al. Entirely subcutaneous defibrillator and complex congenital heart disease: data on long-term clinical follow-up. World J Cardiol 2017;9:547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chang PM, Saxon LA, Doshi RN.. The subcutaneous implantable cardioverter defibrillator as part of dual device therapy in complex congenital heart disease. J Innov Cardiac Rhythm Manag 2016;7:2395–9. [Google Scholar]
- 181. Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG.. Early complications of permanent pacemaker implantation: no difference between dual and single chamber systems. Br Heart J 1995;73:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mounsey JP, Griffith MJ, Tynan M, Gould FK, MacDermott AF, Gold RG. et al. Antibiotic prophylaxis in permanent pacemaker implantation: a prospective randomised trial. Br Heart J 1994;72:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Eberhardt F, Bode F, Bonnemeier H, Boguschewski F, Schlei M, Peters W. et al. Long term complications in single and dual chamber pacing are influenced by surgical experience and patient morbidity. Heart 2005;91:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H. et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest 2004;126:1177–86. [DOI] [PubMed] [Google Scholar]
- 185. Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE.. The relation between patients' outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005;46:1536–40. [DOI] [PubMed] [Google Scholar]
- 186. Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P. et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD database. Circ Arrhythm Electrophysiol 2011;4:136–42. [DOI] [PubMed] [Google Scholar]
- 187. Tobin K, Stewart J, Westveer D, Frumin H.. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000;85:774–6, A779. [DOI] [PubMed] [Google Scholar]
- 188. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC.. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA. et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012;9:728–35. [DOI] [PubMed] [Google Scholar]
- 190. Nowak B, Tasche K, Barnewold L, Heller G, Schmidt B, Bordignon S. et al. Association between hospital procedure volume and early complications after pacemaker implantation: results from a large, unselected, contemporary cohort of the German nationwide obligatory external quality assurance programme. Europace 2015;17:787–93. [DOI] [PubMed] [Google Scholar]
- 191. Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GR. et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J 2004;147:582–6. [DOI] [PubMed] [Google Scholar]
- 192. Sridhar AR, Lavu M, Yarlagadda V, Reddy M, Gunda S, Afzal R. et al. Cardiac implantable electronic device-related infection and extraction trends in the U.S. Pacing Clin Electrophysiol 2017;40:286–93. [DOI] [PubMed] [Google Scholar]
- 193. Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ.. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large us payer database 1-year post implantation. Circ Arrhythm Electrophysiol 2016;9:e003929.. [DOI] [PubMed] [Google Scholar]
- 194. Boriani G, Lane DA, Windecker S, Huber K, Kirchhof P, Lip GY.. Difficult decision making in the management of patients with atrial fibrillation and acute coronary syndrome or invasive cardiovascular interventions: new recommendations for daily practice. Europace 2015;17:1319–22. [DOI] [PubMed] [Google Scholar]
- 195. Boriani G, Burri H, Mantovani LG, Maniadakis N, Leyva F, Kautzner J. et al. Device therapy and hospital reimbursement practices across European countries: a heterogeneous scenario. Europace 2011;13 Suppl 2:ii59–65. [DOI] [PubMed] [Google Scholar]
- 196. Boriani G, Elsner C, Diemberger I.. The struggle against infections of cardiac implantable electrical devices: the burden of costs requires new personalized solutions. Europace 2018;20:1877–9. [DOI] [PubMed] [Google Scholar]
- 197. Boriani G, Maniadakis N, Auricchio A, Muller-Riemenschneider F, Fattore G, Leyva F. et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J 2013;34:1869–74. [DOI] [PubMed] [Google Scholar]
- 198. Maniadakis N, Vardas P, Mantovani LG, Fattore G, Boriani G.. Economic evaluation in cardiology. Europace 2011;13 Suppl 2:ii3–8. [DOI] [PubMed] [Google Scholar]
- 199. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ. et al. ; American Heart Association Committee on Rheumatic Fever Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- 200. da Silva KR, Costa R, Crevelari ES, Lacerda MS, de Moraes Albertini CM, Filho MM. et al. Glocal clinical registries: pacemaker registry design and implementation for global and local integration—methodology and case study. PLoS One 2013;8:e71090.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Weintraub WS, Karlsberg RP, Tcheng JE, Boris JR, Buxton AE, Dove JT. et al. ; American College of Cardiology Foundation, American Heart Association Task Force on Clinical Data Standards. ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards. Circulation 2011;124:103–23. [DOI] [PubMed] [Google Scholar]
- 202. Gliklich RE, Dreyer NA, Leavy MB.. Data collection and quality assurance in. Registries for Evaluating Patient Outcomes: A User's Guide. [Internet]. 3rd edition. Agency for Healthcare Research and Quality (US); 2014 Apr. Report No.: 13(14)-EHC111. [PubMed] [Google Scholar]