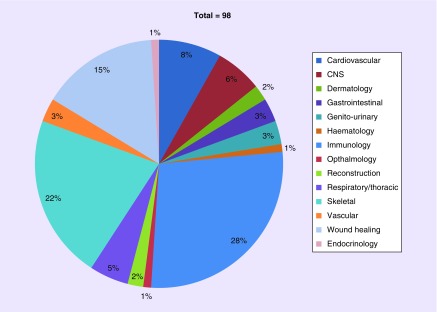
Figure 1. . EU clinical trials involving ‘mesenchymal stem cell’.
A total of 27 (28%) of the 98 mesenchymal stem cell clinical trials currently registered on EudraCT involve immunomodulatory properties of mesenchymal stem cell. A total of 22 (22%) are skeletal applications (bone, tendon repair, osteoarthritis), 15 (15%) address wound healing applications (skin ulcers, burns, fistulae). Cardiovascular (eight trials, 8%) and CNS (six trials, 6%) indications cover the majority of other trials.
Source: EudraCT www.clinicaltrialsregister.eu (Accessed 3 November 2018).