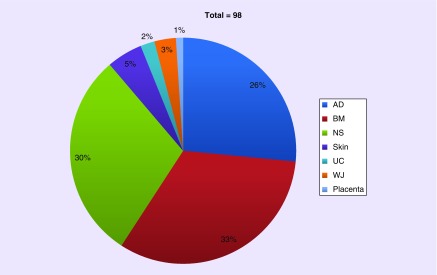
Figure 3. . Tissue sources in EU mesenchymal stromal cells clinical trials.
From the 98 clinical trials involving mesenchymal stromal cell as the investigational medicinal product currently registered on EudraCT, 32 (33%) stated the source of mesenchymal stromal cell as BM, 25 (26%) utilized AD and 29 (30%) did not specify the NS in the primary record or the Competent Authority application form. Skin, UC, W and placenta were also mentioned as source tissues.
Source: EudraCT www.clinicaltrialsregister.eu (Accessed 3 November 2018).
AD: Adipose tissue; BM: Bone marrow; NS: Source tissue; UC: Umbilical cord; WJ: Wharton's jelly.