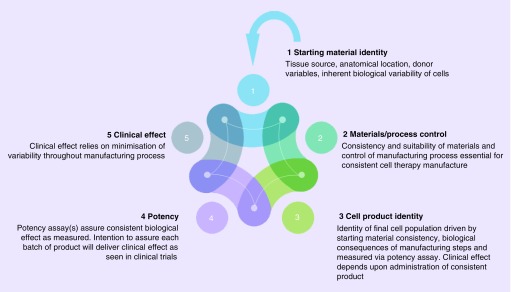
Figure 4. . Identity as an integral part of cell therapy manufacture.
Each aspect of the manufacture of consistent and effective cell therapies is linked: heterogeneity of the starting material (tissue/cell source) is a fundamental source of variability which impacts upon the overall ability of the process to deliver an effective product with consistent relevant biological functionality equivalent to that assessed in clinical trials.