Figure 1.
AAV Gene Therapy
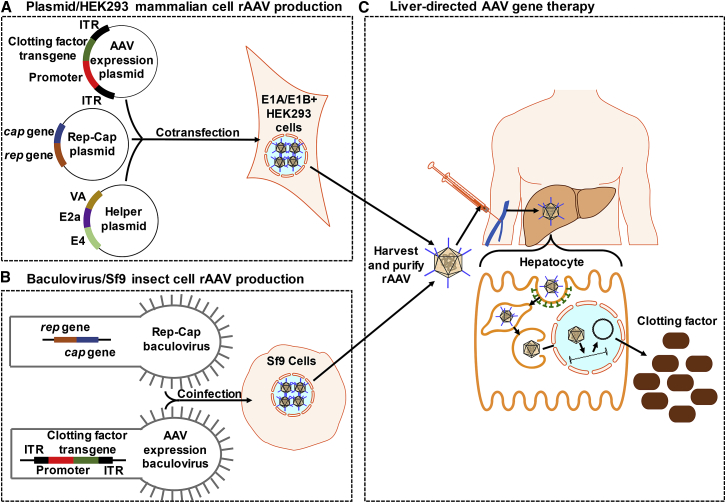
(A) Plasmid/HEK293 mammalian cell rAAV production: HEK293 cells are cotransfected with (1) AAV expression plasmid containing the clotting factor transgene and tissue-specific promoter, flanked by ITRs, (2) plasmid containing the rep and cap genes, and (3) helper plasmid containing adenovirus genes. (B) Baculovirus/Sf9 insect cell rAAV production: Sf9 cells are coinfected with (1) AAV expression baculovirus containing the clotting factor transgene and tissue-specific promoter, flanked by ITRs, and (2) baculovirus containing the rep and cap genes. (C) Liver-directed AAV gene therapy: rAAV is harvested by freeze-thawing transfected/infected cells, purified, and then injected intravenously. The transgene expression is targeted to hepatocytes using a liver-specific promoter and capsid with strong liver tropism. rAAV binds to a serotype-specific host cell receptor and is internalized by a clathrin-coated dynamin-dependent pathway into the endosomal compartment.33, 34, 35, 36 The rAAV is quickly transported using microtubules to the perinuclear region and undergoes conformational changes that expose regions of its capsid to allow for escape from the endosome and nuclear localization signals for trafficking into the nucleus.36, 37, 38 The clotting factor is expressed after AAV uncoats its capsid and converts to a circular double-stranded DNA episome by annealing of plus and minus strands delivered to the same cell or second-strand synthesis using host polymerase.39,40 rAAV, recombinant adeno-associated virus; ITR, inverted terminal repeat.