Figure 3.
Structure and Activity of FVIIIa and Emicizumab
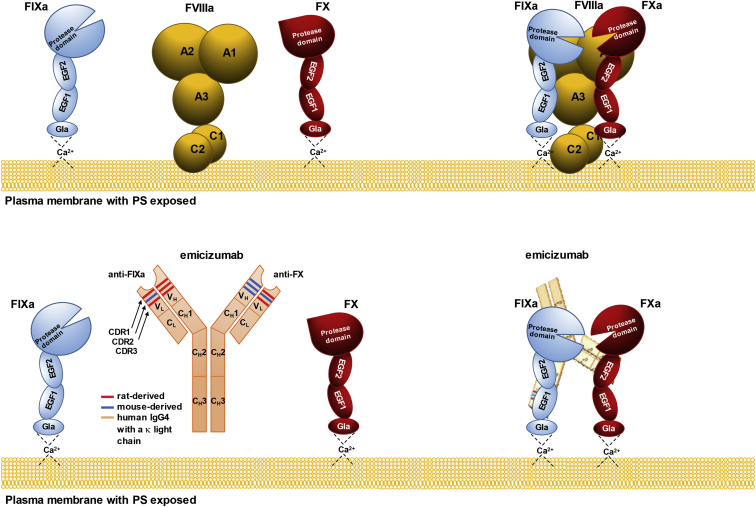
FVIIIa forms a complex with activated FIX (FIXa, a serine protease) and FX (the zymogen), so that FIXa can activate the latter. In this reaction, FVIIIa functions as a molecular scaffold for FIXa, whose protease activity toward FX is otherwise 105- to 106-fold lower and insufficient to drive the thrombin burst and, ultimately, optimal blood coagulation. Emicizumab is an asymmetric humanized bispecific anti-FIXa/anti-FX antibody with mouse and rat complementarity determining regions (CDRs) grafted on a human immunoglobulin (IgG4) with a kappa light chain. While FVIIIa binds multiple sites on FIXa and FX, emicizumab recognizes single epitopes within epidermal growth factor-like domain 1 (EGF1) of FIXa and EGF2 of FX. Unlike FVIIIa, emicizumab does not bind negatively charged phospholipids (e.g., PS [phosphatidylserine]), but their presence is necessary for its procoagulant activity, which suggests that bridging FIXa and FX in proper orientation requires that they sit on the cell surface.47,48