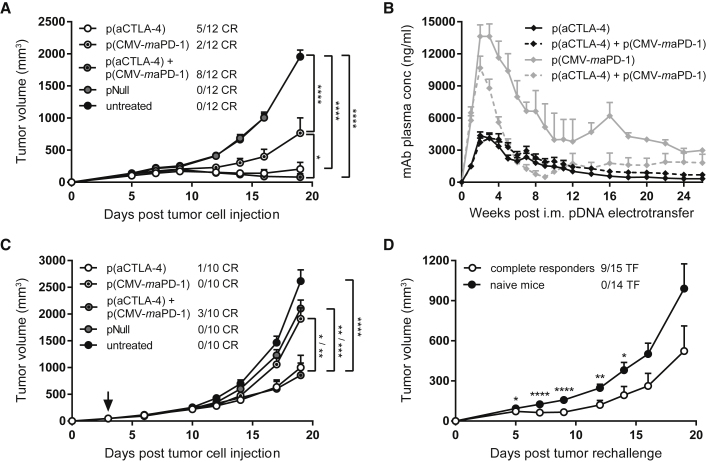
Figure 2.
Prophylactic and Therapeutic Intramuscular Electrotransfer of DNA-Based Checkpoint Inhibitors
C57BL/6J mice received an intramuscular (i.m.) pDNA electrotransfer in the tibialis anterior 7 days before (A, B, and D) or 3 days after (C, indicated by arrow) s.c. MC38 tumor cell injection. One day later, pDNA electrotransfer was repeated in the gastrocnemius muscle. Each electrotransfer, the single-treatment groups received 60 μg p(aCTLA-4) or p(CMV-maPD-1) in the left leg, and the combination-treatment group received both DNA-based mAbs in different legs. Control mice got an equimolar amount of pNull in both legs or were left untreated. (A and C) Tumor growth and number of complete responders (CR). Tumor volumes were compared with one-way ANOVA on day 19 after tumor cell injection, when the first untreated mice had to be sacrificed (n = 10 or 12 mice per group). (B) Anti-CTLA-4 (black lines) and anti-PD-1 (gray lines) mAb plasma concentrations in the single-treatment groups (solid lines) and combination-treatment group (dashed lines; n = 12 mice per group). (D) MC38 tumor rechallenge in complete responders of prophylactic intramuscular DNA-based mAb therapy, 23 weeks after the first tumor cell injection. The number of mice that became tumor-free (TF) after rechallenge is indicated. Tumor volumes were compared with age-matched naive mice with an unpaired t test (n = 14 or 15 mice per group). All data are represented as mean + SEM (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). For Kaplan-Meier survival curves, see Figure S1.