Graphical abstract
Keywords: Protopine, Fumaria indica, HPTLC, Central composite design, DPPH
Abstract
The current study was executed for method development, validation and to estimate the concentration of protopine in methanolic extract of Fumaria indica by high-performance thin-layer chromatography (HPTLC). Isolation of bioactive compounds was carried out using the mobile phase, toluene:ethyl acetate:diethyl amine (8:2.5:0.5 v/v/v), and detected at wavelength 290 nm. This method was validated for precision, specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), etc. The calibration range was found to be 500–5000 ng/spot for the bioactive compound. Protopine was separated with an Rf value of 0.22 ± 0.03. The method was validated for linearity (r2 ≥ 0.996 ± 0.082), accuracy 98.75–102.12%), and RSD of precision (0.49–2.07) with a calibration curve range of 500.00–5000.00 ng/spot. The LOD and LOQ were found to be 83.92 ng/spot and 254.30 ng/spot., respectively. The Central Composite design expert was applied for the validation of robustness. Three independent variables such as the volume of toluene in solvent system, chamber saturation time and wavelength were investigated. The results indicated that a slight change in these variables had no significant effect on the peak response. This developed HPTLC method is simple, precise, robust, specific, rapid, and cost effective. It could be used for quality control study and quantification of protopine in the plant extract and different herbal formulations containing the plant species.
1. Introduction
Plant derived natural drugs are well-known from the start of human civilization for their quality, security, usefulness, cultural suitability and less adverse effects. Because of their natural source, it is assumed that they will show better compatibility with biological systems. Since ancient time numerous herbal plants were utilized as antidiabetic, antioxidant, hepatoprotective, etc. (Amir et al., 2013). Fumaria indica belongs to the family Fumariaceae and called as Haussk in India. It is an annual weed, sub-erect, possibly just scandent and cultivated over the most parts of the plains as well as lower hills of India, Turkey, Iran, Baluchistan and Afghanistan. The whole plant is widely used in traditional and folkloric systems of medicine. This plant is used as anthelmintic, laxative, diuretic, to purify blood, diaphoretic, and in liver obstruction in traditional medicine (Kīrtikara and Basu, 1918, Chopra et al., 1958). Pharmacological evaluation reported that F. indica possesses antidiarrheal (Gilani et al., 2005), anti-inflammatory and anti-nociceptive (Rao et al., 2007), hepatoprotective (Rathi et al., 2008), anxiolytic and central nervous system depressant (Singh and Kumar, 2010), and chemopreventive effect (Hussain et al., 2012). Recently, it has been reported that F. indica is safe and non-toxic through different toxicity analysis (Kumar et al., 2011). This plant showed significant antifungal and antiviral activity (Orhan et al., 2007). Phytochemical study indicated the presence of alkaloids, viz. copticine, fumaritine, fumaramine, fumariline, protopine, parfumine, cryptopine, paprafumicin, paprarine, papracinine, fuyuziphine, narlumidine, narceimine, papraline, fumarophycine, narlumicine; steroids, viz. campesterol, β-sitosterol and stigmasterol; organic acids viz. fumaric acid and caffeic acid (Pandey et al., 2008). The biological activity of Fumaria spp. is generally associated with the presence of isoquinoline alkaloids, the most essential isoquinoline alkaloid found is protopine (Fig. 1). This alkaloid has strong anti-hepatotoxic action (Rathi et al., 2008); inhibits histamine H1-receptors and platelet aggregation (Saeed et al., 1997); inhibits serotonin transporter and noradrenaline transporter, and has an antidepressant (Xu et al., 2006), antimicrobial, antiviral (Orhan et al., 2007) and anti-inflammatory action (Saeed et al., 1997). It was also reported that protopine ingestion down regulated glutamine level in rat brain by activation of glutamate dehydrogenase (Lee et al., 2005) and could assist the fortification against transient cerebral ischemic injury (Hu, 2005).
Fig. 1.
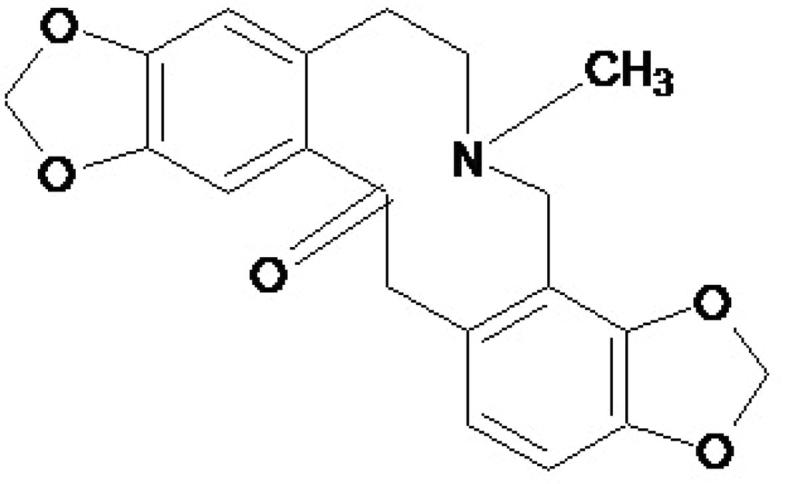
Chemical structure of protopine, MW: 353.4 g/mol.
Response surface methodology (RSM) is a statistical tool, in which several variables are tested simultaneously (Srinubabu et al., 2007, Parajo et al., 1992). The multivariate approach has benefits included decrease in the number of investigational runs, enhances statistical justification potentials and specifies whether parameters interact or not. Central Composite Design (CCD) is widely used statistical experimental design. Although ratability is an anticipated property of a CCD, in the case of difficulty to extending the star points beyond the experimental region defined by the upper and lower limits of each factor (Lundstedt et al., 1998).
Several techniques have been used for the determination of protopine in plant extracts, but until now no study has been reported on HPTLC-densitometry method for quantitative determination of protopine in crude extracts from F. indica. HPTLC-densitometry is a suitable method for screening of huge number of samples for selection of high-producing plants as it is cheaper, faster and simpler than HPLC. Also, the TLC-densitometry method has suitable sensitivity and consistency (Satpathy et al., 2017). The aim of the present study was to develop a HPTLC based detection and quantification of protopine in the extracts of F. indica and to assess the robustness of the chromatographic method for the quantitation of protopine, in the extracts of F. indica, using RSM, and regulate the analytical constraints that present higher effect in the final results of the analysis.
2. Material and methods
2.1. Plant material and reagents
The fresh plant of Fumaria indica was collected from the field area of New Delhi, India in the month of December 2018 and was authenticated by taxonomist, College of Science, King Saud University, Riyadh, Saudi Arabia. Standard protopine (purity: 99.5% w/w) was purchased from Sigma Aldrich, United States. All solvents used were of chromatography grade and all chemicals used were of analytical reagent grade. Precoated silica gel 60 F254 HPTLC plates were purchased from Merck, Darmstadt, Germany.
2.2. Extraction of plant material
The plant of Fumaria indica was dried in sun light properly. The dried plant was powdered using mixer grinder. These crude plant powder (50 g) was defatted separately with 300 mL of petroleum ether by the maceration method. The defatted powder was then extracted with methanol (300 mL) for 6 h by Soxhlet method. This extracts were concentrated by rotary evaporator (Buchi, R-215; Flawil, Switzerland). The concentrated extract was kept in air tight glass container at 5–10 °C for further study. The % extraction yield was calculated by applying the given formula below:
2.3. Preparation of standard stock solution
Stock solution of the reference compound protopine was made by dissolving 10 mg of accurately weighed protopine in 10 mL HPLC grade methanol in a volumetric flask to get a stock solution of 1 mg/mL concentration. This standard stock solution was filtered by 0.45 µm syringe filters and used for HPTLC analysis.
2.4. Preparation of samples
Dried methanolic extract (10 mg) of F. indica extracted by Soxhlet method was dissolve in 100 mL HPLC grade methanol. This sample solution was then filtered by 0.45 µm syringe filters and used for HPTLC study.
2.5. Instrumentation and chromatographic conditions
Chromatographic study was done, as mentioned in previous methods (Lundstedt et al., 1998, Satpathy et al., 2017). Briefly, 20 cm × 10 cm alumina HPTLC plates pre-coated with 200 µm layers of silica gel 60F254 (E. Merck, Darmstadt, Germany) were used for the HPTLC study. Samples were applied as bands 5 mm wide and 10 mm apart by means of Camag Linomat V sample applicator (Muttenz, Switzerland) set with a 100 µL syringe. The stable application rate was 150 nL/s. Linear climbing development with toluene: ethyl acetate: diethyl amine (8: 2.5: 0.5 v/v/v) as solvent system was done in a 20 cm × 10 cm HPTLC chamber formerly saturated with solvent system for 30 min at 60 ± 4% humidity and 25 ± 2 °C temperature. The distance travelled by mobile phase was 80% in near about 10 min. The plates were dried at normal temperature and heated at 110 °C for 5 min to recognize compact bands. Densitometric study was done at 290 nm in reflectance manner with a Camag TLC scanner III controlled through WinCATS software (Version 1.2.0). The slit dimensions were 4 mm × 0.45 mm and the scanning speed of 15 mm/s.
2.6. Validation of HPTLC method
The developed HPTLC process was validated with respect to the following Parameters. The validation process was done as per the ICH guideline (ICH, 2005).
2.6.1. Linearity
Standard solution of protopine was ready in methanol to obtain a concentration of 1 mg/mL. Different quantities of standard solution were selected for application on the TLC plate to obtain an absolute dilution of 500–5000 ng/spot for protopine. All concentration was applied six times on the TLC plate. This TLC plate was then developed by the previously mentioned solvent system. Calibration curves were obtained by plotting the peak area verses different concentrations of the protopine. Linear standard plot was made by least-squares linear-regression study.
2.6.2. Specificity
The capability of an analytical procedure to clearly assess the analyte in the presence of other components can be verified by evaluating specificity. The specificity of the HPTLC process was determined by analyzing standard drug and test samples. The spot for protopine in the samples was confirmed by comparing the Rf value and peak of the spot to that of a standard. The peak purity of protopine was determined by comparing the peak at three various regions of the spot that is peak start, peak apex and peak end.
2.6.3. Precision
According to the guidelines of ICH, precision should be analyzed at two phase, inter-day and intra-day precision. Inter-day precision observes to applying the analytical method in different days over a specific period of time by the same analyst with unchanged instrument. Intra-day was determined by the applying of analytical method within a day at different period of time by the same analyst with the same instrument.
2.6.4. Accuracy
To evaluate the consistency and appropriateness of the developed procedure, recovery studies were performed. This study was performed by standard adding procedure. Known quantity of reference marker protopine was added to pre-studied samples and its recovery was matched with the theoretical value.
2.6.5. Robustness by design expert
A Central Composite statistical screening design (Parajo et al., 1992), was applied to optimize the compositional parameters and to estimate quadratic effects of the toluene volume in solvent system (mL), chamber saturation time (min) and wavelength (nm). Seventeen experiments with three center points were performed by selection of three factors, toluene volume in solvent system (A), chamber saturation time (B), wavelength (C) and peak area was chosen as the response. The supposed value of these three factors A, B and C was 8 mL, 30 min and 290 nm; respectively. The statistics generated were examined via Design-Expert (Version 11.0.3.0, Stat-Ease Inc., Minneapolis, MN, USA) statistical software. The significance of the factors was calculated by using Fisher's statistical test for Analysis of Variance (ANOVA) model that were expected. These components were then used to calculate an F-ratio that estimates the usefulness of the model. If the F-ratio possibility is low, the model is considered a better statistical robust for that data. Every experiment was carried out in randomized order to reduce the bias effects of uncontrolled factors.
2.6.6. Limit of detection and limit of quantification
The limit of detection (LOD) and the limit of quantification (LOQ) were estimated by the blank determination process. In order to determine the LOD and LOQ, blank methanol was spotted six times and the area calculated. The signal-to noise level was estimated. LOD was considered as 3:1 and LOQ as 10:1.
2.6.7. Estimation of protopine in plant sample
The amount of protopine in F. indica was examined via developed and validated procedure by calibration curve. The plant extract solution was applied in triplicates on the TLC plate and area of each triplicate samples were used for study of quantity of marker by linearity equation. The results of triplicate evaluation were uttered as mean quantity of protopine in % w/w.
2.7. Assessment of % free radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH)-UV method
The free radical scavenging power of the plant sample was observed using DPPH-UV method (Mujeeb et al., 2012). DPPH (0.004%) was freshly dissolved in methanol and used as the control. The DPPH solution (1 mL) was added to a mixture of 1 mL of plant extract (10 mg/mL) and 3 mL of methanol. Quercetin (1 mg/mL) was used as a standard and follow the same procedure as per plant extract (Mujeeb et al., 2012). The solution mixtures were shaken vigorously and kept at 20 °C in the dark light for 10 min. The decline in absorbance of the resulting solutions were measured at 517 nm after 10 min. The decrease of absorbance between the control and the plant extract/standard was applied for calculating the % free radical scavenging activity. Each result was examined in triplicate.
where AC = Absorbance of control, AS = Absorbance of sample.
3. Results and discussion
3.1. Extraction
The quantity of plant extract that an extraction yields in a specific solvent is frequently an approximate assess of the quantity of particular compound that the plant material contains. The extraction process was done by Soxhlet method by using methanol as solvent. During the study, time of extraction, temperature of extraction and plant sample: solvent ratio remained constant. The % extractive yield of F. indica was found to be 9.41 ± 0.096% w/w.
3.2. Optimization of method
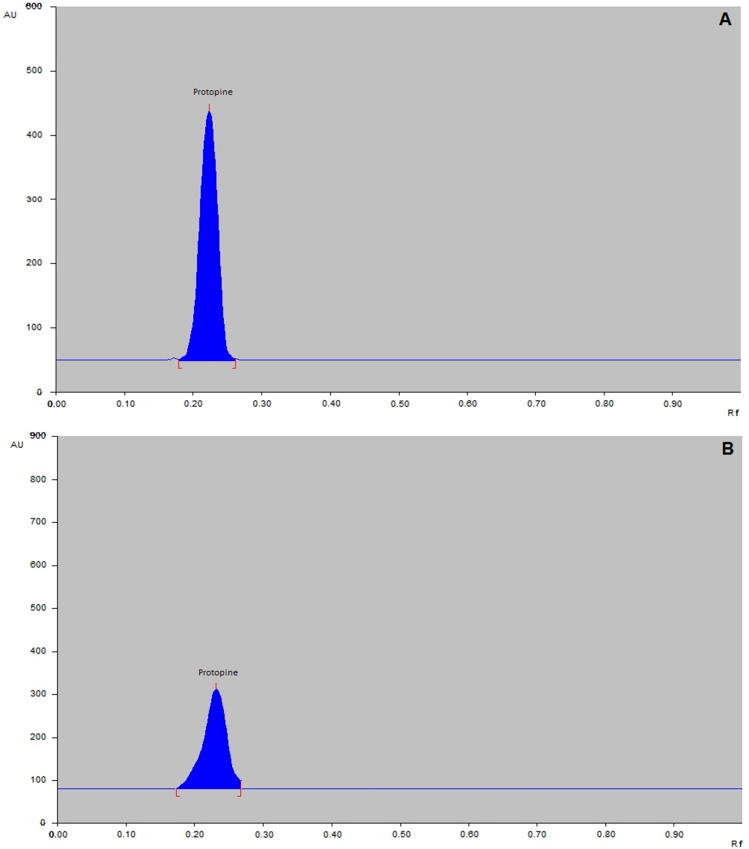
TLC or HPTLC is mainly used as an economical technique for isolation, separation, qualitative detection, or semi-quantitative visual study of samples. Accordingly, TLC is generally described as a pilot technique for HPLC (Rozylo and Janicka, 1996). However, now a day the TLC and HPTLC method can be applied to solve numerous qualitative and quantitative analytical problems in an extensive range of fields, like environmental analysis, biotechnology, medical science, food as well as nutrition analysis, toxicology, biochemistry, chemistry and pharmaceutical science (Weins and Hauck, 1996) utilization of TLC/HPTLC has extended significantly because of the improvement of forced flow (FF) and gradient TLC techniques, advance stationary phase and selection of solvent system, as well as development of new methods for quantification (Poole and Poole, 1994). Composition of mobile phase and ratio of solvents were studied as variables to optimize the chromatographic circumstances. The chromatograms were recorded as well as the Rf value and resolution were calculated. The chromatographic conditions for HPTLC like detection of wavelength and solvent system composition were optimized to give perfect, exact and reproducible results for the analysis of protopine. A scanning wavelength of 290 nm for protopine was obtained from UV spectrum. An excellent resolution was achieved by using an optimum solvent system consisted of toluene:ethyl acetate:diethyl amine (8.0:2.5:0.5 v/v/v). Protopine was adequately resolved with Rf value at 0.22 ± 0.03 (Fig. 2).
Fig. 2.
(A) Densitogram of standard Protopine (1000 ng; Rf = 0.22 ± 0.03); (B): sample extract (Rf = 0.22 ± 0.06).
3.3. Method validation
3.3.1. Linearity
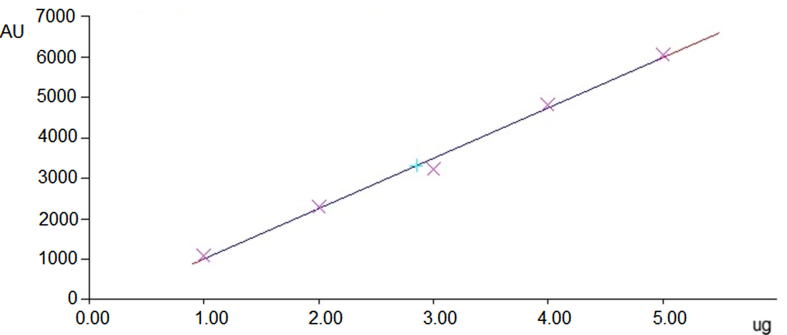
The linearity of an analytical procedure is its facility to draw analysis results that are directly, or by means of precise mathematical alteration, proportional to the concentration of standard in samples within a set range. The linear regression data found for the standard plot (n = 6) explained an outstanding linear relationship over different concentration level 500–5000 ng/spot for protopine (Fig. 3) with respect to area of chromatogram. The plot shows the y-intercept values were low and standard deviations of slope were also quite low. The r2 value was found to be 0.996 ± 0.082.
Fig. 3.
Calibration plot with respect to peak area by HPTLC at different concentration levels of standard protopine.
3.3.2. Specificity
Assessment of analyte in the occurrence of interference to ascertain peak purity in sample chromatogram is a very complex task. Specificity is a determination of the degree of interference from other active ingredients, degradation materials, impurities and additives. Specificity in a process ensures that a peak response is because of a single component only. Specificity is commonly calculated and documented in a separation by the resolution and tailing factor. The specificity of the method was determined by analyzing the standard drug and extract. The spot of protopine in the plant sample was proved by comparing the Rf values and peak of the spot with that of the standard.
3.3.3. Precision
The precision of an analytical procedure is the level of agreement among particular test results achieved when the process is useful to numerous sampling of a homogenous sample. It is an evaluation of the reproducibility of the complete analytical method under standard working conditions. The precision of sample application and observation of chromatogram area were showed in conditions of %RSD and results are illustrated in Table 1 that exposed inter-day and intra-day difference of protopine at three various dilution points of 2000, 3000 and 4000 ng/spot.
Table 1.
Inter-day and intra-day precision of the HPTLC method (n = 6) for protopine (mean ± SD).
| Amount (ng/spot) | Inter-day precision |
Intra-day precision |
||
|---|---|---|---|---|
| Mean peak area ± SD | %RSD | Mean peak area ± SD | %RSD | |
| Protopine | ||||
| 2000 | 2476.08 ± 47.61 | 1.923175 | 2461.35 ± 51.07 | 2.07 |
| 3000 | 3474.35 ± 17.14 | 0.49335 | 3459.60 ± 26.23 | 0.75 |
| 4000 | 4876.58 ± 70.17 | 1.438938 | 4887.93 ± 52.84 | 1.08 |
RSD: Regressed Standard Deviation.
3.3.4. Accuracy
Accuracy articulated the closeness of understanding between the values which is established as an ordinary genuine value or an established reference value and the observed obtained. This test permits the evaluation of probable bias. The selected method of HPTLC was used for the assortment of accurate mobile phase for estimation of protopine in selected herbal drug after injecting via 50%, 100% and 150% of added references standard, which showed best recovery of 98.75–102.12%. The results of % of drug recovered and % RSD are shown in Table 2.
Table 2.
Accuracy of the HPTLC method (n = 6) for Protopine (mean ± SD).
| % Of standard spiked to the sample | Theoretical content (ng) | Amount of drug recovered ng ± SD | % of drug recovered | % RSD |
|---|---|---|---|---|
| Protopine | ||||
| 0 | 2500 | 2539.35 ± 19.24 | 101.57 | 0.75 |
| 50 | 3750 | 3746.03 ± 24.18 | 99.89 | 0.64 |
| 100 | 5000 | 5106.14 ± 98.66 | 102.12 | 1.93 |
| 150 | 6250 | 6172.22 ± 65.99 | 98.75 | 1.06 |
RSD: Regressed Standard Deviation.
3.3.5. Robustness by design expert
The robustness of an analytical method is a measure of its ability to stay unaffected by introduction of small discrepancies in method constraints and provides assign of its consistency through-out normal procedure. For measuring robustness of the method, a number of chromatographic parameters, such as, flow rate, detection wavelength, injection volume, compositions in mobile phases, or column temperature are varied within a convincing range and quantitative influence of the variables is examined or determined. The robustness of current developed method was examined/tested by using the CCD. The multivariate approach using design of experiments was used in robustness testing, in order to study the concurrent dissimilarity of the factors on the responses of peak areas of protopine. RSM is an effective statistical and mathematical procedure to examine and optimize composite processes (Bezerra et al., 2008). RSM provides a large amount of information with less number of experiments for observing the interface of the free variables on the response. CCD is broadly employed because of its extraordinary competence with respect to the conventional practices that required large number of scores for sample analysis or optimization (Srinivas et al., 2008, Wang et al., 2006). A CCD is a two factorial design involves 2 k factorial runs with ±α beside each variable bloc, and at least one center point (Srinubabu et al., 2007). Center point replications (more than two) are mostly carried out to allow the estimation of pure error of the response within the calculated range and to check the analytical validity of the model (Gunst et al., 1996). The varieties examined were small deviations from the method settings and the parallel responses in the retention time considered were observed.
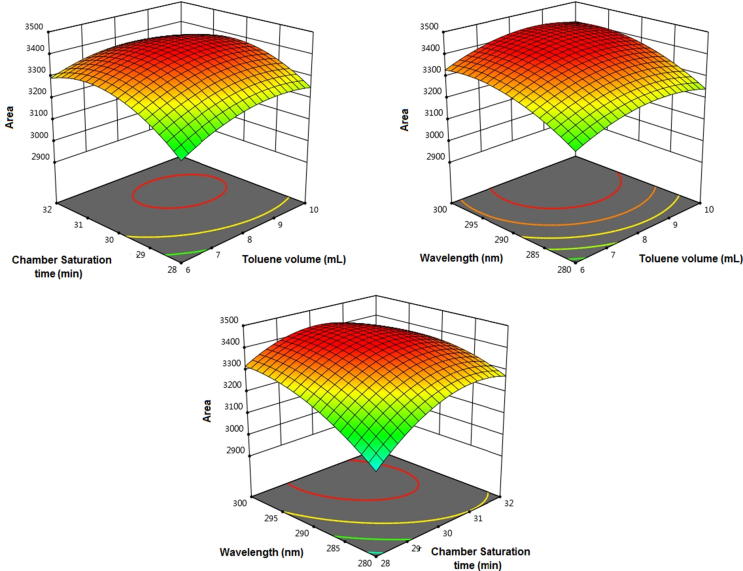
A three-factorial, Central Composite statistical experimental design was carried out by 17 experiments test run. The independent variables and the responses for all 17 optimized test experimental runs are illustrated in Table 3. It was studied that the best-fitted model was the quadratic model and the relative values of SD and % CV for the various projected models. Only statistically significant (p < 0.05) coefficients are incorporated in the equations. A positive value represents an effect that favors the optimization, although a negative value indicates an opposite connection between the factor and the response (Gunst et al., 1996). It is clear from the equation that the factor toluene volume of mobile phase (A) as well as wavelength (C) has a positive effect and chamber saturation time (B) has a negative effect on the response (Y). The red color intensity represents the concentration of protopine (Fig. 4). It also confirms that the association between responses and factors is not constantly linear. Used at different levels in study or when more than one factor is changed simultaneously, a factor can produce different degrees of response. For an experimental design with three variable factors, the appropriate model fitting to the data was the quadratic model. The polynomial equations for the response factors are given below:
Table 3.
Chromatographic factors for central composite response surface methodology.
| Run | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| A:Toluene volume (mL) | B:Chamber saturation time (minute) | C:Wavelength (nm) | |
| 1 | 8 | 26.63 | 290 |
| 2 | 6 | 32 | 280 |
| 3 | 8 | 30 | 273.18 |
| 4 | 8 | 33.36 | 290 |
| 5 | 4.63 | 30 | 290 |
| 6 | 8 | 30 | 306.81 |
| 7 | 10 | 28 | 280 |
| 8 | 8 | 30 | 290 |
| 9 | 8 | 30 | 290 |
| 10 | 10 | 32 | 300 |
| 11 | 10 | 32 | 280 |
| 12 | 11.36 | 30 | 290 |
| 13 | 6 | 32 | 300 |
| 14 | 6 | 28 | 300 |
| 15 | 8 | 30 | 290 |
| 16 | 6 | 28 | 280 |
| 17 | 10 | 28 | 300 |
Fig. 4.
Response-surface 3D graphs showing the effect of (A) Chamber saturation time (min) versus toluene volume (ml) and wavelength remains constant (B) wavelength (nm) versus toluene volume (ml) percentage and Chamber saturation time (min) remains constant (C) wavelength (nm) versus Chamber saturation time (min) and toluene volume (ml) remains constant.
Peak Area (Y) = +3408.64 + 33.34*A + 54.20*B + 76.36*C − 22.04*A*B + 0.5200*A*C − 48.61*B*C − 58.94*A2 − 98.89*B2 − 61.13*C2. Where A is the toluene volume of mobile phase (mL), B is the chamber saturation time (min) and C is the wavelength (nm).
The data was examined by analysis of variance (ANOVA). The reliability of the fitted model was assessed by the determination co-efficient (R2 = 0.9889), adjusted (R2a = 0.9799) and (CV = 1.36%). A very high adjusted determination coefficient (R2a = 0.969) values confirmed that the model was very significant (Parajo et al., 1992). In the present research study, values of R2a were found to be contiguous to the R2. High values of R2 = 0.9889 presented an outstanding relationship between the tested and projected responses (Lundstedt et al., 1998). The lower CV value indicated that the abnormalities between tested and predicted values are low. Adequate precision was found to be 16.89 and greater than 4 is desirable in the design, confirms this design is significant for determination robustness in chromatography (Beg et al., 2003).
3.3.6. Limit of detection and limit of quantification
The limit of detection (LOD) is explained as the lowest amount of a reference standard in a sample that can be detected, but not essentially quantify. It is a limit test that indicates whether a reference standard is high or lowers a specific value. The limit of quantification (LOQ) is explained as the lowest amount of a reference standard in a sample that can be quantified with suitable accuracy and precision below the confirmed operational circumstances of the process. In order to analyze the LOD and LOQ, blank methanol was spotted six times. The signal-to-noise level was examined. LOD was measured as 3:1 and LOQ as 10:1. The LOD with S/N ratio of 3:1 was obtained 83.92 ng/spot and LOQ with S/N ratio of 10:1 was obtained 254.30 ng/spot.
3.3.7. Estimation of protopine in plant sample
The amount of protopine in the extract of Fumaria indica was determined via developed and validated chromatographic method by calibration curve. A well-defined and well resolved sharp peak of protopine was observed at the Rf value of 0.22 ± 0.02 in the chromatograms of the sample extracted from F. indica. The protopine content in the plant of F. indica was found to be 3.69 ± 0.46% w/w, with low % RSD value specified the appropriateness of this process for regular study of protopine in F. indica during the formulation development.
3.4. Assessment of % free radical scavenging activity by DPPH-UV method
DPPH-UV method is used for quick evaluation of pure active antioxidant constituent in complex mixtures, mainly herbal extracts. Interest in the research for natural antioxidants has grown over the past years because reactive oxygen species (ROS) production and oxidative stress have been shown to be linked to diseases such as cancer, cardiovascular disease, osteoporosis, and degenerative diseases. Natural antioxidant substances are believed to play a potential role in interfering with the oxidation process by reacting with free radicals, chelating catalytic metals and scavenging oxygen in biological systems (Amir et al., 2013). The faster the absorbance reduces; the more potent antioxidant efficacy of the constituent is in terms of hydrogen-donating ability (Shirwaikar et al., 2006). This study proved that the F. indica plant extract samples have the proton giving capability and could work as free radical inhibitors or scavengers, acting probably as antioxidants. It was observed that the extract of F. indica at dilution of 10 mg/mL had free radical scavenging activity of 78.91 ± 0.926% which is comparable to that of the standard quercetin (1 mg/mL) (93.21 ± 0.625%). This result confirms that the method can be useful for a rapid screening of antioxidant potential of compounds or radical scavenging activity of phytoconstituents.
4. Conclusion
A validated HPTLC analytical method has been developed for the analysis of protopine in F. indica. The projected method is accurate, simple, precise, less time consuming, cost effective and has the capability to isolate the active compound from its other constituents. It also has good linearity range and limits of determination. Application of factorial design using CCD for validation of robustness testing showed that a slight change in tested parameters. However, a special consideration is prerequisite for firm monitoring of the aforesaid tested factors during chromatographic testing. Therefore, the developed method is proved to be rapid, simple, and selective and could be used for routine analysis of protopine in F. indica in bulk as well as in pharmaceutical formulations containing protopine.
Acknowledgments
Acknowledgements
The author would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding through research group no- (RG#1435-017).
Declaration of Competing Interest
The authors have confirmed that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amir M., Mujeeb M., Ahmad S., Akhtar M., Ashraf K. Design expert-supported development and validation of HPTLC method: an application in simultaneous estimation of quercetin and rutin in Punica granatum, Tamarindus indica and Prunus domestica. Pharm. Methods. 2013;4(2):62–67. [Google Scholar]
- Beg Q.K., Sahai V., Gupta R. Statistical media optimization and alkaline protease production from Bacillus mojavensis in a bioreactor. Process Biochem. 2003;39(2):203–209. [Google Scholar]
- Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra I.C. Glossary of Indian medicinal plants. Quart. Rev. Biol. 1958;33(2):156. [Google Scholar]
- Gilani A.H., Bashir S., Janbaz K.H., Khan A. Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J. Ethnopharmacol. 2005;96(3):585–589. doi: 10.1016/j.jep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Gunst R.F., Myers R.H., Montgomery D.C. Response surface methodology: process and product optimization using designed experiments. Technometrics. 1996;38(3):285. [Google Scholar]
- Hu X.-Y. Effects of Corydalis ambailis migo total alkaloids on experimental cerebral ischemia. J. Chinese Integrative Med. 2005;3(1):46–49. doi: 10.3736/jcim20050114. [DOI] [PubMed] [Google Scholar]
- Hussain T., Siddiqui H.H., Fareed S., Vijayakumar M., Rao C.V. Evaluation of chemopreventive effect of Fumaria indica against N-nitrosodiethylamine and CCl4-induced hepatocellular carcinoma in Wistar rats. Asian Pacific J. Trop. Med. 2012;5(8):623–629. doi: 10.1016/S1995-7645(12)60128-X. [DOI] [PubMed] [Google Scholar]
- ICH, 2005. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Wiley StatsRef: Statistics Reference Online. John Wiley & Sons, Ltd.
- Kīrtikara, K.R., Basu, B.D., 1918. Indian medicinal plants, Sudhindra Nath Basu, Pâninî office; [etc., etc.].
- Kumar V., Chauhan S., Singh G., Rai G. Fumaria indica is safe during chronic toxicity and cytotoxicity: a preclinical study. J. Pharm. Pharm. 2011;2(3):191. doi: 10.4103/0976-500X.83287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Huh J.-W., Choi M.-M., Yoon S.Y., Yang S.-J., Hong H.-N., Cho S.-W. Regulation of glutamate level in rat brain through activation of glutamate dehydrogenase by Corydalis ternata. Exp. Mol. Med. 2005;37(4):371–377. doi: 10.1038/emm.2005.47. [DOI] [PubMed] [Google Scholar]
- Lundstedt T., Seifert E., Abramo L., Thelin B., Nyström Å., Pettersen J., Bergman R. Experimental design and optimization. Chemometr. Intell. Lab. Syst. 1998;42(1–2):3–40. [Google Scholar]
- Mujeeb M., Ashraf K., Aqil M., Amir M., Khan A., Sharma D. Phytochemical analysis and in vitro antioxidant activity of Uncaria gambir. Int. J. Green Pharm. 2012;6(1):67. [Google Scholar]
- Orhan I., Özçelik B., Karaoğlu T., Şener B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from fumaria and corydalis species. Zeitschrift für Naturforschung C. 2007;62(1–2):19–26. doi: 10.1515/znc-2007-1-204. [DOI] [PubMed] [Google Scholar]
- Pandey M.B., Singh A.K., Singh J.P., Singh V.P., Pandey V.B. Fuyuziphine, a new alkaloid fromFumaria indica. Nat. Prod. Res. 2008;22(6):533–536. doi: 10.1080/14786410701592596. [DOI] [PubMed] [Google Scholar]
- Parajo J.C., Alonso J.L., Lage M.A., Vazquez D. Empirical modeling of eucalyptus wood processing. Bioprocess. Eng. 1992;8(3–4):129–136. [Google Scholar]
- Poole C.F., Poole S.K. Instrumental thin-layer chromatography. Anal. Chem. 1994;66(1):27A–37A. [Google Scholar]
- Rao C., Verma A., Gupta P., Vijayakumar M. Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharm. 2007;57(4) doi: 10.2478/v10007-007-0039-z. [DOI] [PubMed] [Google Scholar]
- Rathi A., Srivastava A.K., Shirwaikar A., Singh Rawat A.K., Mehrotra S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine. 2008;15(6–7):470–477. doi: 10.1016/j.phymed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Rozylo J.K., Janicka M. Different planar techniques for prediction of solute retention in column liquid chromatography. J. Planar Chromatogr. 1996;9(6):418–424. [Google Scholar]
- Saeed S.A., Gilani A.H., Majoo R.U., Shah B.H. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol. Res. 1997;36(1):1–7. doi: 10.1006/phrs.1997.0195. [DOI] [PubMed] [Google Scholar]
- Satpathy S., Patra A., Hussain M.D., Ahirwar B. Simultaneous estimation of genistein and daidzein in Pueraria tuberosa (Willd.) DC by validated high-performance thin-layer chromatography (HPTLC) densitometry method. J. Liq. Chromatogr. Relat. Technol. 2017;40(10):499–505. [Google Scholar]
- Shirwaikar A., Prabhu K.S., Punitha I.S.R. In vitro antioxidant studies of Sphaeranthusindicus (Linn) Indian J. Exp. Biol. 2006;44:993–996. [PubMed] [Google Scholar]
- Singh G.K., Kumar V. Neuropharmacological screening and lack of antidepressant activity of standardised extract of Fumaria indica: l study. E. J. Pharmacol. Ther. 2010;3:19–28. [Google Scholar]
- Srinivas, S.K., Narasimha, R.M., Allam Appa R., Maheswari, I.L., Srinubabu, G., 2008. Development and validation of LC method for the determination of leflunomide in pharmaceutical formulations using an experimental design. AfrJ Pure Appl. Chem. 2.
- Srinubabu G., Raju C.A.I., Sarath N., Kumar P.K., Rao J.V.L.N.S. Development and validation of a HPLC method for the determination of voriconazole in pharmaceutical formulation using an experimental design. Talanta. 2007;71(3):1424–1429. doi: 10.1016/j.talanta.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Wang Y., Harrison M., Clark B.J. Optimising reversed-phase liquid chromatographic separation of an acidic mixture on a monolithic stationary phase with the aid of response surface methodology and experimental design. J. Chromatogr. A. 2006;1105(1–2):199–207. doi: 10.1016/j.chroma.2005.11.101. [DOI] [PubMed] [Google Scholar]
- Weins C., Hauck H. Advances and developments in thin layer chromatography. LC-GC Int. 1996;14(6):455–471. [Google Scholar]
- Xu L.-F., Chu W.-J., Qing X.-Y., Li S., Wang X.-S., Qing G.-W., Fei J., Guo L.-H. Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models. Neuropharmacology. 2006;50(8):934–940. doi: 10.1016/j.neuropharm.2006.01.003. [DOI] [PubMed] [Google Scholar]