Figure 4.
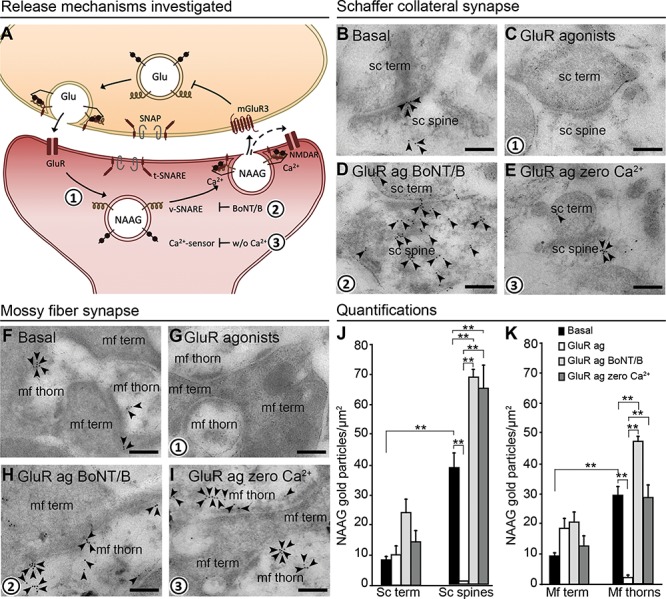
NAAG is depleted by exocytosis from postsynaptic dendrites. The schematic drawing (A) shows the proposed mechanism of action and regulation of NAAG release (numbers in bold indicate mechanisms studied by us). Presynaptic membrane fusion of transmitter-containing vesicles is mediated by members of the SNARE family: the v-SNARE, target membrane SNARE (t-SNARE), and synaptosomal-associated protein (SNAP), in addition to Ca2+ sensors. We investigated whether the same mechanism is responsible for the postsynaptic NAAG release. Glutamate released from the nerve terminal stimulates dendritic ionotropic glutamate receptors (GluR) (1), which triggers Ca2+-dependent (2) and v-SNARE-dependent (3) release of NAAG. NAAG then acts on presynaptic mGluR3/postsynaptic NMDAR (mixed agonist/antagonist), the former inhibiting Glu release from the nerve terminal. The electron micrographs (B–I) show how inhibiting exocytosis during membrane depolarization affects the depletion of NAAG immunogold particles from postsynaptic dendritic spines/thorns. During basal conditions (2.5 mM K+), gold particles representing NAAG were present postsynaptically in dendritic spines and thorns belonging to Schaffer collaterals (B, sc spine) and mossy fibers (F, mf thorn) and with very low levels in the presynaptic terminals (sc term and mf term). When postsynaptic depolarization was elicited by GluR agonists, NAAG was depleted from the postsynaptic spines (C) and thorns (G). When v-SNARE was degraded by BoNT/B, NAAG was trapped postsynaptically despite GluR agonist activation (D and H). Finally, in the absence of Ca2+ (zero Ca2+), NAAG was also trapped postsynaptically despite the presence of GluR agonists (E and I). Scale bars, 100 nm. Quantitative results of NAAG immunogold particles in different tissue profiles are shown in (J) for the Schaffer collateral synapses of the stratum radiatum and (K) for the mossy fiber synapses of the dentate gyrus. Resting conditons; 2.5 mM K+ (basal, black bars), GluR agonists (GluR ag., white bars), GluR agonists in the presence of botulinum toxin B (GluR ag BoNT/B, light gray bars), and GluR agonists in the absence of Ca2+ (GluR ag zero Ca2+, dark gray bars). The bars represent the average number of NAAG immunogold particles/μm2 + SD in Schaffer collateral spines (sc spines) and nerve terminals (sc term) belonging to Schaffer collateral synapses in the stratum radiatum (J), and thorns (mf thorns) and mossy fiber terminals (mf term) belonging to hilar mossy fiber synapses (K). **: P < 0.01, one-way ANOVA, Tukey’s multiple comparisons test, GraphPad. The numbers of tissue profiles per slice that was included in the quantifications were as follows: sc spines and sc terminals: 20–26 (basal), 23–29 (GluR agonists), 21–25 (GluR agonists zero Ca2+), 19–25 (GluR agonists BoNT/B); mf thorns: 37–63 (basal), 41–71 (GluR agonists), 53–57 (GluR agonists zero Ca2+), 47–73 (GluR agonists BoNT/B); mf terminals: 19–25 (basal), 20–29 (GluR agonists), 20–21 (GluR agonists zero Ca2+), 19–22 (GluR agonists BoNT/B), (slice experiment from two rats (pooled), n = 4–5 slices in each condition).
