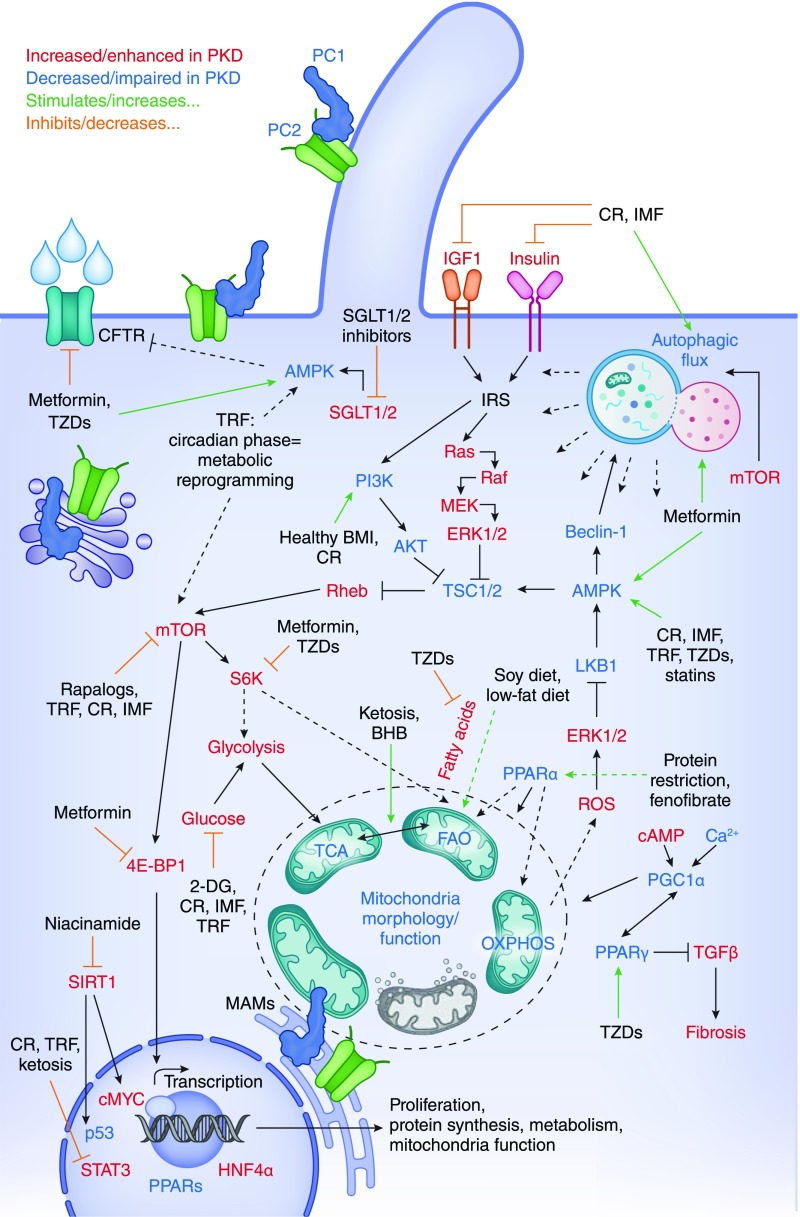
Figure 1.
Autosomal dominant polycystic kidney disease (ADPKD) and nutrient metabolisms have converging pathways. Central cellular processes that are known to be impaired in ADPKD pathology and are characteristic of metabolic reprogramming include autophagic flux, glycolysis, fatty acid oxidation, and mitochondrial function. Central signaling nodes that overlap between ADPKD and metabolic response include mammalian target of rapamycin (mTOR), AMP-activated kinase (AMPK), sirtuin-1 (SIRT-1), IGF-I, and peroxisome proliferator–activated receptor-α/γ (PPARα/γ). Alteration in diet intake or composition can affect many of these overlapping processes/pathways. Similarly, multiple pharmacologic approaches that are known to alter metabolic reprogramming target these central processes/signaling hubs. Collectively, this suggests that such interventions have high potential in alleviating ADPKD in humans. Dotted arrows indicate that signaling cascade is more complex than depicted. AKT, protein kinase B; BHB, β-hydroxybutyrate; BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; cMYC, cellular mycelocytomatosis; CR, caloric restriction; 2-DG, 2-deoxyglucose; 4E-BP1, eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; ERK1/2, extracellular signal–regulated kinase; FAO, fatty acid oxidation; HNF4α, hepatocyte nuclear factor 4α; IMF, intermittent fasting; IRS, insulin receptor substrate; MAM, mitochondria associated membranes; MEK, mitogen-activated protein kinase kinase; OXPHOS, oxidative phosphorylation; PC, polycystin; PI3K, phosphoinositide 3-kinase; Rheb, ras homolog enriched in brain; ROS, reactive oxygen species; S6K, ribosomal protein S6 kinase; SGLT1/2, sodium glucose cotransporter-1/2; STAT3, signal transducer and activator of transcription 3; TCA, tricarboxylic acid cycle; TRF, time-restricted feeding; TSC1/2, tuberous sclerosis 1/2; TZD, thiazolidinedione.