FIG. 4.
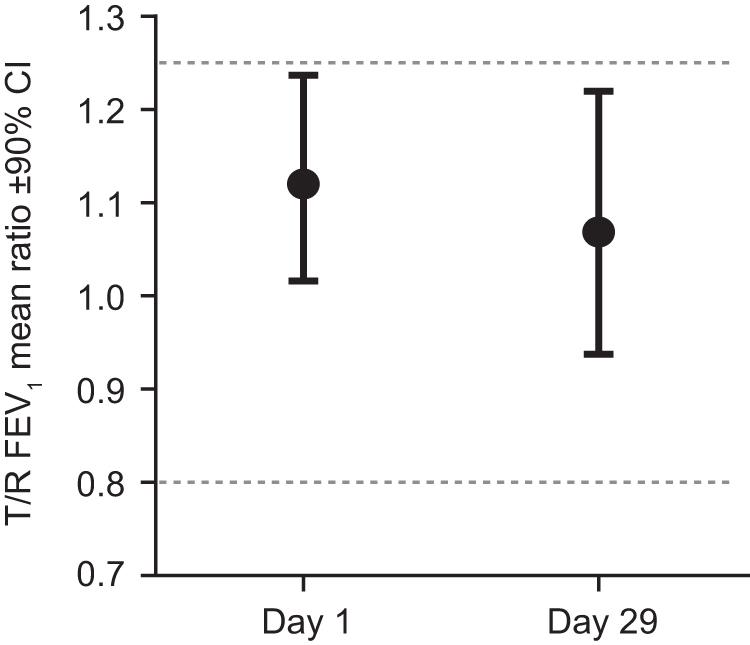
Day 1 and day 29 bioequivalence test. T/R FEV1 LS mean ratio and 90% CI for both day 1 (FEV1 AUC(0–12)) and day 29 (trough FEV1) co-primary endpoints were within the standard bioequivalence limits, shown as dotted lines. AUC(0–12), area under the effect curve over 12 hours postdose; R, reference product (Advair Diskus); T, test product (Wixela Inhub).
