Abstract
Microbial communities are complex multi-species assemblages that are characterized by a multitude of interspecies interactions, which can range from mutualism to competition. The overall sign and strength of interspecies interactions have important consequences for emergent community-level properties such as productivity and stability. It is not well understood how interspecies interactions change over evolutionary timescales. Here, we review the empirical evidence that evolution is an important driver of microbial community properties and dynamics on timescales that have traditionally been regarded as purely ecological. Next, we briefly discuss different modelling approaches to study evolution of communities, emphasizing the similarities and differences between evolutionary and ecological perspectives. We then propose a simple conceptual model for the evolution of interspecies interactions in communities. Specifically, we propose that to understand the evolution of interspecies interactions, it is important to distinguish between direct and indirect fitness effects of a mutation. We predict that in well-mixed environments, traits will be selected exclusively for their direct fitness effects, while in spatially structured environments, traits may also be selected for their indirect fitness effects. Selection of indirectly beneficial traits should result in an increase in interaction strength over time, while selection of directly beneficial traits should not have such a systematic effect. We tested our intuitions using a simple quantitative model and found support for our hypotheses. The next step will be to test these hypotheses experimentally and provide input for a more refined version of the model in turn, thus closing the scientific cycle of models and experiments.
This article is part of the theme issue ‘Conceptual challenges in microbial community ecology’.
Keywords: microbial communities, interspecific interactions, experimental evolution, mathematical modelling, spatial structure, mutualism
1. Introduction
Microorganisms play key roles in biogeochemical cycling, industry, and health and disease of humans, animals and plants [1–5]. For example, the roughly 1030 microbial cells on our planet contain 10 times more nitrogen than all plants combined and are responsible for half of the global production of O2 [6]. Almost all of these microorganisms reside in communities, which are assemblages of multiple interacting species. Despite their importance, we currently know little about how microbial communities form and function. However, such knowledge is crucial if we want to fundamentally understand their properties and dynamics, as well as control the processes that they mediate [7,8].
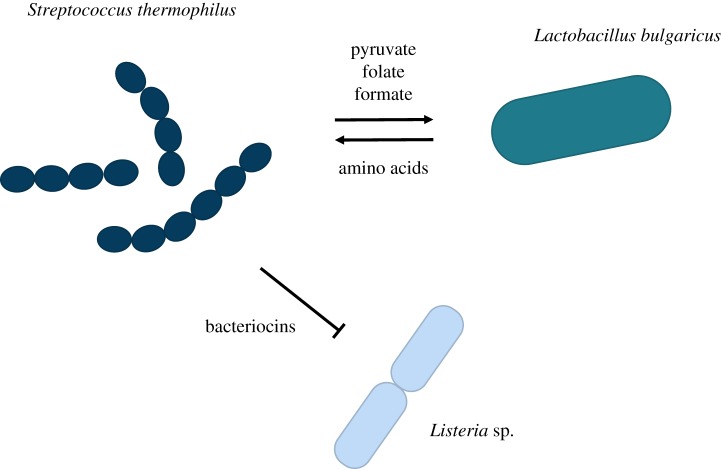
Microbial communities, like all complex systems, are more than the sum of their parts: they are characterized by a multitude of often complex interactions between their constituent members (figure 1). At any given time, microbes may compete for shared resources such as metabolites and space, inhibit each other via the secretion of antibiotics and other toxic compounds, and even kill each other upon direct cell–cell contact [11,12]. Yet, not all is bleak in the microbial world: some organisms may—accidentally or actively—excrete enzymes or molecules that others can use, and even commit suicide for others [13–15]. The overall sign and strength of an interaction between two organisms is the net result of all such processes and can be characterized as anything from competition to parasitism to mutualism [16,17].
Figure 1.
Microbial communities are characterized by a multitude of often complex interactions between their members. For example, three bacterial species that can co-occur in dairy products engage in both positive and negative interactions, which are mediated by metabolic compounds and toxins [9,10]. (Online version in colour.)
Interspecific interactions have a crucial role to play in microbial communities. That is, what makes a community a community—rather than a random set of species—is precisely these interactions, because they give rise to properties at the level of the community that we cannot understand by considering each species in isolation. For example, it may not be possible to predict from growing each species by itself what the joint reproductive output of a collective will be, or how robust such a collective will be to external biotic and abiotic perturbations. Microbial communities can also perform chemical transformations that would be impossible for one individual species to achieve [18], and some communities even display complex behaviours such as collective motion and electrochemical signalling, which have traditionally been associated with higher organisms [19,20].
Over the past decades, an impressive amount of effort has been dedicated towards obtaining an ever-more detailed and realistic picture of a wide range of different microbial systems. The advance of -omics technologies has led to an incredible leap forward in terms of available data that can be integrated to extract and analyse patterns that inform us about the lives of microbes in their natural surroundings. However, if we want to arrive at a more fundamental understanding of the current and future properties of microbial communities, we will have to uncover general principles of how such communities typically change over time.
Since interactions are often mediated by whole suites of different chemicals, interactions may significantly change in strength and even sign over ecological timescales. For example, bacteria may alter the pH of their environment during growth, which may then change the sign of their interactions with other species from positive to negative, or vice versa [21]. Extrapolating such findings to more general principles of community dynamics is challenging. Nonetheless, an increasing number of studies find that ecological community dynamics are remarkably repeatable across experimental and biological replicates, and may be understood from a combination of metabolic properties of the environment and species functional traits [22–24]. Less is known about how communities change over evolutionary timescales, even if such changes have potentially much more far-reaching consequences because they are less likely to reverse (because they are the result of genetic mutations). Given that interactions are the defining feature of a community, we propose that how interspecific interactions evolve is most important in this respect.
Here, we review the empirical evidence that evolution is an important driver of microbial community properties and dynamics on timescales that have traditionally been regarded as purely ecological. Next, we briefly discuss different modelling approaches to this problem, emphasizing the similarities and differences between evolutionary and ecological perspectives. We then propose a simple conceptual model for the evolution of communities, which we explore using simulations. Finally, we discuss experimental approaches that may help to test our framework and thus improve our understanding of this fascinating process.
2. Evolution of microbial communities: empirical approaches
The question of how microbial communities evolve has been addressed empirically by authors from several different fields. One approach that has substantially increased in popularity over recent years is genomic analysis of one or more—often pathogenic—species that evolve within a host environment, such as the gut or the cystic fibrosis lung [25]. Some of these studies employ temporal metagenomics to capture the entire diversity present within a given population, whereas others use culture-based population genomics to obtain more reliable estimates of the phylogenetic relationship between genetic variants [26,27]. This group of studies has revealed that evolution in the microbiome can be fast and highly repeatable [28], with 109–1012 new single-nucleotide polymorphisms arising on a daily basis [27], de novo mutations competing for extended periods of time [29], and gene gains and losses sweeping to high frequencies within a period of just a few months [30].
Metagenomic approaches have also been used to study evolution in microbial communities that are not host-associated. A 9-year study of biofilm communities from acid mine drainage allowed the authors to estimate the in situ nucleotide substitution rate for a focal species and revealed several divergence and hybridization events [31]. A similar study performed on a freshwater lake community monitored multiple species simultaneously and found evidence for extensive within-population genetic heterogeneity, as well as selective sweeps in some species but not others [32]. Horizontal gene transfer also appears to be an important driver of adaptation in natural communities, at least when they are exposed to selective pressures such as antibiotics or heavy metals [33,34]. Finally, metagenomic approaches have been used in conjunction with phylogenetic tools to infer evolutionary rates of microbial communities as a whole over longer timescales [35]. These studies underline the importance of the environment for the rate of adaptation, with communities evolving most slowly in energy-limited environments that impede growth [36] and evolving most rapidly in extreme environments that impose strong selection [37].
Another field that is concerned with the evolution of microbial communities is artificial community selection. This approach entails the repeated selection of microbial communities from a larger pool based on their performance with respect to some a priori defined community-level function, such as productivity or enzyme production [38–40]. Given that this approach does not usually assess genetic changes in each of the constituent species, it is conceivable that the observed changes are mainly due to ecological sorting rather than evolutionary adaptation [41,42]. However, the duration of these experiments is sufficient for evolution to occur, and such short-term evolution can directly impact ecological sorting [43]. Moreover, it has been argued that continued species coexistence and within-species evolution are crucial for maximally effective community selection [44]. One important advantage of this approach over methods that are centred on focal species is that it explicitly tracks the dynamics of the entire community. As such, the success or failure of this approach may provide valuable insights into whether we can regard communities as a unit of selection [45–48] and to what extent we can predict changes in community-level properties over time.
A more bottom-up approach to studying the evolution of microbial communities is laboratory evolution. This method involves culturing replicate populations of organisms under controlled conditions for long periods of time, while probing the resulting phenotypic and genotypic changes [49,50]. While this method has been used mainly to look at the evolution of single species, an increasing number of studies employ simple two-species communities to ask how species evolve in the presence of a coevolutionary partner [51]. Mostly, these experiments focus on the evolution of species with a predefined interspecific relationship—such as host–parasite [52], predator–prey [53] or mutualism [54,55]—and assess how interaction traits like parasite infectivity and host defence change over evolutionary time. At least two studies explicitly report a change in the nature of the interactions between two species: in one case, a commensal interaction quickly evolved into exploitation [56], whereas in the other case, ammensalism evolved into antagonism [57].
Given the increasing popularity of laboratory evolution as a tool and the fact that natural communities often consist of a large number of interacting species, surprisingly few studies have extended the above approaches to communities of more than two species (but note [58], as well as an increasing body of work that asks how focal species evolve in the presence of a community [59–62]). One important exception is a study in which five species from a beech tree hole were cocultured in the laboratory for approximately 70 generations [63]. Characterization of the evolved communities revealed that species had diverged in resource use and evolved to feed on by-products excreted by the other species. As a result, interspecific interactions became more positive (in the sense that growing species in pairs leads to a higher yield than would be predicted based on growing them individually), and community productivity increased. The same qualitative results were also recovered when between 1 and 12 species from the same model community were evolved under three different conditions [64], again demonstrating the importance of studying the evolution of microbial communities, rather than evolution in microbial communities.
Taken together, the above approaches provide us with a wealth of data on the evolution of microbial communities, which give us a first rough understanding of the nature of this process. The emerging picture is that evolution is likely to be an important driver of community dynamics and properties on what have traditionally been regarded as ecological timescales [65–67]. One major scientific challenge—and opportunity—is now to develop a framework that allows us to predict evolutionary changes in microbial communities, based on theoretical and mechanistic principles. Our goal here is to contribute to the development of such a framework.
3. Evolution of microbial communities: modelling approaches
To test our intuitions about general principles of community evolution, one powerful approach is to use mathematical and computational models. Such models allow us to abstract a system to its minimal components and assess how different features of the system interact, ideally generating testable hypotheses that can be addressed using carefully designed experiments [68,69]. In particular, they allow us to test a wider range of possibilities, such as environmental conditions or combinations of species, than would be experimentally feasible.
There are many models describing aspects of the evolution of microbial communities, but few of these models consider the process in its entirety. This is not surprising given that community evolution is a complex problem that requires an understanding of microbiology as well as a combination of modelling approaches from evolution and ecology, two disciplines that have traditionally been poorly integrated despite their conceptual similarities. However, comparing approaches from both fields and combining the relevant elements from each of them may be a promising strategy towards tackling this problem. To this end, we here briefly review the main properties of some common evolutionary and ecological models and evaluate their usefulness for understanding the evolution of microbial communities, identify their major limitations, and discuss how to extend current modelling approaches to be able to better predict evolution of communities.
(a). Evolutionary models
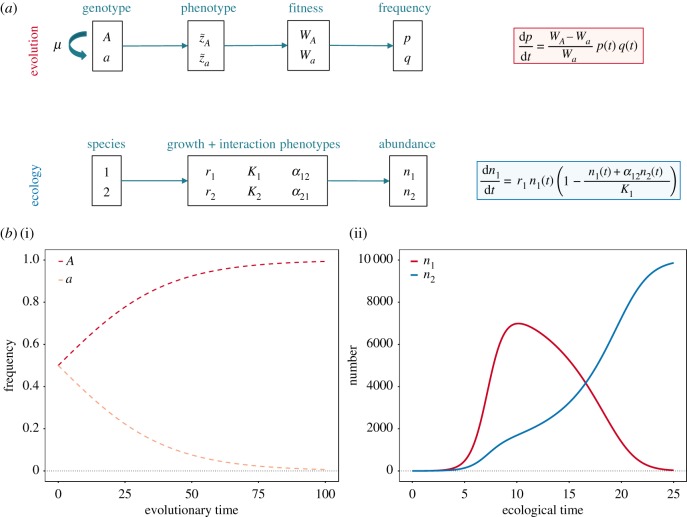
One important class of evolutionary models is population genetics models. These models are generally focused on tracking changes in genotype or allele frequencies over long timescales (figure 2) [70]. Different genotypes arise via mutations or migration and map to some measure of reproductive success, or fitness [71], perhaps via an intermediate phenotype. The genotype with the highest fitness increases in frequency until it eventually replaces all other types or is outcompeted by new mutations with even higher fitness [72]. Many variations on this theme exist with added levels of complexity, such as recombination [73], spatial structure [74] and more complex demography [75].
Figure 2.
Conceptual set-up of classical models in evolution and ecology. (a) Population genetics models consider different genotypes that can interconvert via mutation (μ). Each genotype maps to some fitness (W) value, perhaps through an intermediate phenotype (z). A mathematical model (red box) then describes the change in frequencies of the genotypes (p and q) over long timescales, where the average speed of this process depends on the relative fitness of each type. By contrast, ecological models consider multiple species, each of which maps to a set of growth and interaction phenotypes (r, K, α). A mathematical model (blue box) tracks the changes in abundances of different species (n1 and n2) over shorter timescales. (b) (i) Dynamics of genotype frequencies from the evolutionary model (pt0 = qt0 = 0.5, WA = 1.05, Wa = 1.00). (ii) Dynamics of species abundances from the ecological model ( r1 = 1.25, r2 = 1.00, K1 = 10 000, K2 = 10 000, α12 = 1.00, α21 = 1.75).
By contrast, quantitative genetic models typically focus on traits encoded by a large number of loci, which are assumed to evolve independently owing to substantial recombination, as is common in animals and plants [76]. Therefore, these models ignore the underlying genetics of the phenotype and instead track the change in the mean phenotype (z) of the population over time. Populations are composed of many different types that each has a different deviation from the mean, as well as a different fitness. Selection then acts on this variation to increase population fitness. The overall relationship between phenotype and fitness is crucial: larger total variation in phenotype and larger selection on that phenotype lead to faster evolutionary change [77,78].
Because evolutionary models typically consider dynamics over long timescales, the model parameters such as fitness or population sizes are usually some average values that integrate over many underlying phenomena. For example, the fitness may be an average fitness over many possible environments that fluctuate among each other or over many stages of life history. As a result, it can be difficult to link these models to explicit biological mechanisms. In particular, explicit interactions between organisms and their biotic and abiotic environment are almost always neglected in these models. The reason is that the dynamics of these interactions are assumed to be much faster than evolutionary dynamics and hence can be averaged out. However, as aforementioned, it is increasingly clear that ecological and evolutionary dynamics often occur on similar timescales in microbes. Therefore, an explicit treatment of ecological processes should be necessary to more accurately describe evolution, particularly when considering evolution in the context of microbial communities.
(b). Ecological models
Arguably the most classic ecological model is the Lotka–Volterra model (figure 2) [79]. This model tracks over time the abundance of two or more different species, which are assumed to be immutable. When the species are growing far from their carrying capacity K, or maximum population size at which the population has a positive growth rate, the abundances increase exponentially and independently between species. However, as the population sizes increase, intraspecific and interspecific interactions reduce growth, such that the species with the fastest maximum growth rate may not dominate in the long run. An advantage of ecological models is that they make explicit possible trade-offs between life-history traits: for example, when organisms are growing in quickly changing, resource-rich environments far from carrying capacity (e.g. frequent migration into new resource patches), it pays off to grow faster, but when growing in constant, resource-poor environments close to carrying capacity (e.g. biofilms), it pays off to be more efficient or have a more inhibiting effect on your competitor.
The Lotka–Volterra model is purely phenomenological, i.e. it describes the dynamics of the process without making any direct reference to the underlying mechanisms [79]. By contrast, consumer–resource models explicitly track the resources that are responsible for the observed dynamics [80]. Such an added level of detail can help to understand why we observe certain dynamics and make predictions beyond the current observations. It is even possible for phenomenological and mechanistic models to generate opposite predictions about the dynamics of the system for relatively simple cases [81]. However, for each additional quantity that is tracked over time, additional parameters are needed, and in the case of microbial communities, where many chemically mediated interactions occur simultaneously, keeping track of and explicitly modelling each of these interactions is daunting.
One approach that can potentially overcome this problem is community-level metabolic modelling known as flux balance analysis, where the metabolic conversions performed by each species in the community are predicted from the species' genomes, under the assumption that each cell maximizes its growth rate (i.e. the rate of biomass production) [82]. While simpler versions of these models assume that each of the individual species is in steady state, more complex versions allow for variation in metabolic flux depending on the environment, and thus the tracking of the abundance of each individual species over time [8].
A second important extension of these models is the incorporation of spatial structure [83]. Given that most microbes live in spatially structured environments [84] and chemicals can only travel limited distances [85], it is clear that microbes do not usually interact equally with everyone else in the population, but instead interact most strongly with their immediate neighbours [13]. As such, growth rate as well as interaction parameters such as K and α will vary over space and time. This may lead to locally different outcomes of competition, which can have important consequences for the reproductive success of each type within the population as a whole. Such local variations in population dynamics and their global consequences are commonly explored using individual-based simulations [86,87], where each cell grows, dies and moves depending on its local context. The advantage of such approaches is that they can explore the emergent properties of complex systems where many factors interact, especially for cases where analytical solutions to the problem cannot be obtained.
(c). Integrating evolution and ecology
Considering the classes of models reviewed above, it is clear that for a conceptual and computational model to adequately capture the evolution of microbial communities, it will have to incorporate elements from both evolution and ecology. We need a model that describes which mutations get selected and at what rate, but because interactions in communities are complex and often changing, a single fitness value will generally fail to predict the long-term evolutionary fate of a mutation. Also, we are dealing with multiple mutable species, and this element is missing from traditional evolutionary models. Some processes, such as mutation or kin selection [88], are particular to within-species dynamics. However, many other elements from evolution and ecology can in principle be used interchangeably, as the distinction between different genotypes of the same species and different species is often arbitrary. Taken together, a model suiting our purpose would have to allow for mutation and selection, as well as intra- and interspecific interactions. Additionally, because interactions are often local and mediated by chemicals, spatial and mechanistic models may be more accurate in many situations compared with mass-action and phenomenological models. Finally, for a model to capture the evolutionary dynamics of the community as a whole, it will have to allow not only for the evolution of each of the individual species, but also for the evolution of the interactions between them.
Some models exist already at this intersection of evolution and ecology. One emerging discipline is eco-evolutionary dynamics, which focuses on the interplay between the composition of species and the abundance of these species [65,67,89,90]. However, the most notable category of interest for the current problem is evolutionary game theory [91–94], where the fitness of a phenotype depends on the frequency of the other phenotypes in the population. These models do precisely what we are looking for: they describe which mutations get selected at which rate, and allow for fitness to depend on the biotic context. Indeed, the mathematical description of these models is very similar to that of the Lotka–Volterra equation [95], even if frequency- and density-dependence do not necessarily have the same consequences (consider, for example, the two time points in figure 2b(ii) where the two types have equal frequencies but different abundances: after time 0, n1 increases in frequency, but after time 17, n2 increases in frequency). Game-theoretical models are often used to investigate how costly cooperative traits within species (the production of ‘public goods’), such as the secretion of extracellular enzymes that catalyse the transformation of resources [96] or toxins that kill a competing strain or species [97,98], can evolve or be maintained in the presence of defecting or cheating individuals that do not pay the cost but gain the benefit. Sometimes they also incorporate mutation [99] to explore how the mean trait level within a species evolves over time. This approach has also been applied to the evolution of interspecific interactions [100,101], although the underlying traits are often different in that case [102].
(d). Evolution of interspecific interactions
Most modelling approaches that assess how interspecific interactions change over evolutionary timescales focus on one specific type of interaction, such as interference competition [103] (where two species interact in a way that is detrimental for both species, an interaction that can be represented as ‘−/−’), host–parasite [104,105] or predator–prey interactions [106] (+/−), or mutualism [107,108] (+/+). Generally, these models look at how a single trait such as parasite infectivity or host defence changes over time by considering different types (which may or may not be generated by mutation) within each species, how their frequencies change over time, and what consequences this has for the population as a whole.
Understanding how such traits evolve is important when studying the evolution of microbial communities. However, not all microbial interactions can be classified with such simple schemes, and the nature and strength of ecological interactions often change with time [21,109,110]. Moreover, even when the nature of an interaction is stable, this does not necessarily mean that the phenotypic traits on which the interaction is based will evolve, because species may adapt to their local biotic and abiotic environment in myriad other ways, particularly when the community consists of more than two species. Therefore, the question is not whether such interspecific interactions can evolve, but what the likelihood relative to other traits is that they will evolve.
One useful framework to structure our thinking about this question is the classical Hamiltonian scheme that categorizes a behaviour based on its effect on both the actor and the recipient (which in this case are two different species). This scheme distinguishes four different classes: mutual benefit (effect on self: +/ effect on other: +), selfishness (+/−), altruism (−/+) and spite (−/−) [14,111,112]. The selection of mutations that fall within the first two classes is easily explained, because they provide a direct fitness benefit to the actor. However, the selection of mutations that fall within the latter two classes is more counterintuitive. Why would an organism do something that is bad for itself? Often, this is explained by kin selection: when suffering a cost yourself is offset by a sufficiently large benefit to another individual that is likely to share the same cooperative gene (for example, because it is a relative), the net consequence of the behaviour may still be positive for this gene, so that it will be positively selected [88,113].
However, when considering behaviours that affect other species, this explanation does not apply by definition, because the recipient is highly unlikely to share the cooperative gene. An alternative explanation is reciprocity, where the recipient is likely to return the ‘favour’, be it positive or negative [100,114,115]. Examples of traits that fall into these categories are the secretion of a costly metabolic compound for a mutualistic species, or the production of an antibiotic that inhibits an antagonistic species. Mutations conferring such traits may be regarded as conferring an indirect fitness benefit to the actor. Because they are selected for their effect on the recipient rather than their effect on the actor (which is negative), such mutations have a disproportionate chance of changing the strength or nature of interspecific interactions, and as such, will be particularly relevant for the evolution of community-level properties.
4. Conceptual and computational models for the evolution of microbial communities
As indicated above, reciprocity is required for a mutation with an indirect fitness benefit to be positively selected. How can such reciprocity be achieved? Any situation where the recipient is sufficiently more likely to return the ‘favour’ specifically to the actor or its direct descendants, rather than to all individuals of the acting species in general, will have such a consequence [115–119]. In the case of higher organisms, this may occur because of complex behaviours such as the recipient remembering who previously did it a favour [120,121]. However, in microbes, the easiest way for reciprocity to occur is when they live in a spatially structured environment [100,122–126].
To see this, consider the fate of a mutant that produces a costly antibiotic that kills a competing species. When such a mutant arises in a well-mixed environment, such as a shaking flask, all its conspecifics will now be able to grow faster because their competitor is inhibited, while they do not pay the cost of antibiotic production. As a consequence, they will grow more rapidly than the mutant. The mutant will thus not be able to increase in frequency within its own population. By contrast, when the same mutant arises in a spatially structured environment, such as a biofilm, the mutant and its descendants will profit more than their average conspecific from the inhibition of their neighbouring competitors. This is because the inhibitory compounds, as well as the extra nutrients that are now available, are locally more concentrated. In spatially structured environments, such a mutant is expected to increase in frequency within its population, given that the associated cost is not too high.
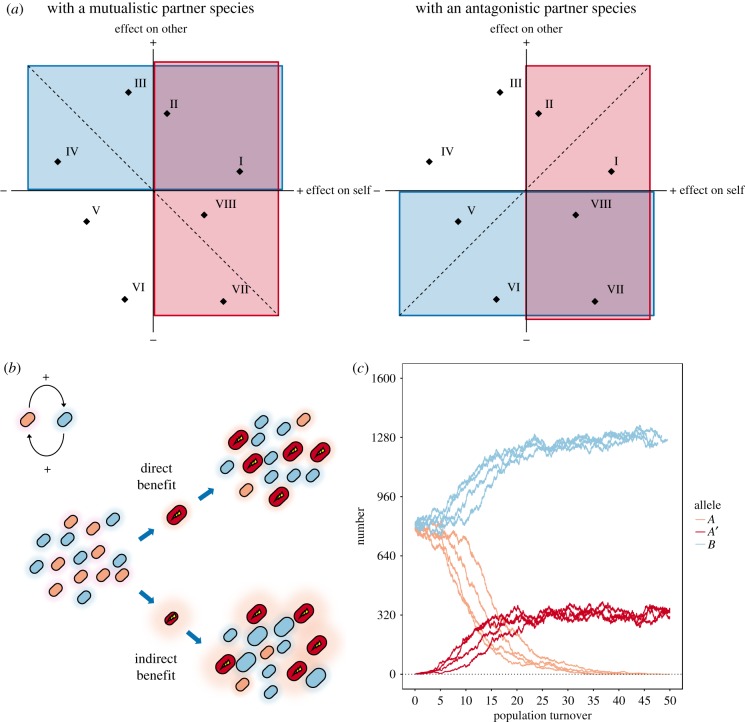
Based on the above argument, we hypothesize that evolution in well-mixed environments will proceed exclusively via the selection of directly beneficial traits. By contrast, evolution in spatially structured environments may proceed via the selection of both directly and indirectly beneficial traits (figure 3a); which of these two modes predominates will depend on the availability and effect size of mutations on each type of trait. Importantly, mutations that provide a direct fitness benefit may also affect other organisms in both positive and negative ways as a pleiotropic effect. For example, when an organism becomes better at using a resource and collaterally produces more metabolic by-products, others may also profit from this. However, such a mutation will not be selected because of the effect that it has on others, but because of the effect that it has on the actor itself.
Figure 3.
A conceptual and computational model for the evolution of microbial communities. (a) Schematic depicting the hypothesis put forward in this paper. Mutations may be selected because they have a positive effect on the individual itself (red boxes), and/or because they have a positive effect on a mutualistic species or a negative effect on an antagonistic species (blue boxes). When selection acts only on mutations with direct effects (in well-mixed environments), mutations that have a positive effect on the individual itself can increase in frequency (left and right panels: mutations I, II, VII and VIII). When selection acts on mutations with both direct and indirect effects (in spatially structured environments), mutations that have a net positive effect can increase in frequency. For example, in the left panel mutations to the top and right of the dashed—hypothetical trade-off—line (I, II, III and VIII) are associated with larger benefits than costs and may thus be positively selected. Conversely, mutations to the bottom and left of the dotted line (IV, V, VI and VII) are associated with larger costs than benefits and will thus be negatively selected. In the right panel, mutations to the bottom and right of the dotted line (I, VI, VII and VIII) may be positively selected, and mutations to the top and left of the dotted line (II, III, IV and V) will be negatively selected. This implies that in some cases mutations with direct fitness costs may be selected for, and mutations with direct fitness benefits selected against. (b) We modelled the case where two species (red and blue) have a positive initial effect on each other. Red individuals can evolve by the acquisition of two types of mutations. First, they may acquire mutations that provide a direct fitness benefit, such as becoming better at using a certain resource, or becoming more tolerant to a toxic compound. Individuals carrying such mutations should be able to increase in frequency in both spatially structured and well-mixed environments. Second, they may acquire mutations that provide an indirect fitness benefit, such as producing a costly compound that increases the growth of the blue species. Because the blue species has a positive effect on the red species, having more blue cells around, as well as each of these cells having a higher growth rate, also provides a fitness benefit to the red species (e.g. because the blue cells excrete a metabolic by-product that the red species can use, at a rate proportional to their growth rate). Individuals carrying such mutations should only be able to increase in frequency in spatially structured environments. Cell size reflects growth rate. (c) Population dynamics in 10 replicate runs of a simple cellular automaton model, simulating a spatially structured environment. Alleles A and B grow better when there are more cells of the other type in their immediate neighbourhood. A costly, more cooperative A′ mutant (conceptually equivalent to mutation III in the left panel of a) invaded in 4/10 simulations and reached an intermediate equilibrium frequency. By contrast, this mutant invaded in 0/10 replicates of a model simulating a well-mixed environment.
To test the idea that spatial structure will determine whether mutations with indirect benefits are selected for, we developed a simple cellular automaton model [97] that incorporates elements from both evolution and ecology: it allows for intra- and interspecific interactions as well as the possibility of these interactions evolving by mutations. This model is similar to previous work that analysed the evolution of traits that determine interspecies interactions (e.g. [100,124,125]) but with an important distinction—that it explicitly distinguishes between direct and indirect fitness effects of mutations (which can both be either positive or negative). We assume that cells of two different species, A and B, grow together on a 40 × 40 grid with periodic boundary conditions. The fitness of each cell depends on the fraction of cells around it of each type of species, as well as the fitness of each of these neighbouring cells. So, an interaction impacts both the reproductive success of the other species and its reciprocal interaction. In each time step, one cell in the grid reproduces with a probability proportional to its fitness. We then randomly choose another cell to die, with the descendant of the reproducing cell pushing all cells in its path towards the now-empty spot vacated by the dead cell. A complete population turnover will take on average 40 × 40 = 1600 such time steps. For details of the model, see the Methods section.
Here we focus on the scenario where both types already have a slightly positive effect on each other (i.e. an example of passive mutualism [123]; figure 3b). We assess whether a single mutant A’, which has a more positive effect on B but grows more slowly than the other A cells, can increase in frequency within the population. Note that, even if we do not explicitly test this here, we predict that the same logic will also apply to the case where both species have a slightly negative effect on each other and a costly mutant with a stronger negative effect emerges. We run the simulation for 50 whole-population turnovers and determine the probability that the mutant establishes, i.e. that it rises above some minimum abundance so that it is unlikely to be lost by stochastic effects. In parallel, we run control simulations for a well-mixed environment, where the fitness of a cell depends not just on its neighbours, but on all other cells in the population.
For the parameter settings that we chose, we find that a costly mutant with a strong positive effect on a cooperating species invades in 41 out of 100 cases (figure 3c, Methods). When it does, the original A genotype is driven to extinction, and the mutant reaches an equilibrium concentration of approximately 20% (that is, A′ equilibrates at a lower frequency than the other type, B, presumably because it invests into an interaction that is beneficial for B). By contrast, in the well-mixed environment, the mutant invades in only 2 out of 100 cases, and when it does, it reaches only very low, fluctuating concentrations, suggesting that such invasion is the result of genetic drift. While the precise dynamics depend on the details of the model as well as the parameter settings [127], our goal here is to provide a proof of principle showing that costly mutations that provide an indirect fitness benefit can be selected for in a multi-species community. Models are abstractions, and as such will never be able to capture the full complexity of the real world. However, they serve an important purpose in isolating and directly evaluating the importance of specific factors for the phenomena that we observe. When we find that our intuitions bear out in simple—arguably unrealistic—cases, we can then start to explore which conditions are necessary for these findings to hold, and how different individually important factors interact.
Our results are consistent with previous models that investigated the effect of spatial structure on the evolution of mutualism [100,124,125]. However, our model is different in that it does not focus exclusively on a single, cooperative trait or interaction, but can be easily adjusted to consider the selection of selfish or antagonistic traits. An interesting future extension of the model might be to allow for mutants providing direct and indirect fitness benefits to arise simultaneously, and to investigate how the success of such mutants depends on the composition of each species as well as their abundances. For example, based on the models discussed above, we might predict that mutations with a direct fitness benefit will have a relative advantage at lower densities, because they increase growth rate (r), whereas mutations with an indirect fitness benefit will have a relative advantage at higher densities, because they impact interactions (α). Another interesting extension is to investigate the impact of genetic architecture and the correlation structure of mutational effects on self versus non-self. That is, are mutations with a direct versus indirect fitness benefit more likely to occur, and/or have a different distribution of fitness effects? Does a mutation with a more positive effect on the actor generally have a more positive effect on others around it as well?
Similarly, it might be insightful to explicitly model the different classes of metabolites that are expected to underlie the interactions and to assess whether such a mechanistic approach makes predictions that are qualitatively different from those of the phenomenological approach that we used here. Finally, real communities consist of more than two species (or multiple strains of the same species, which should have similar consequences as long as these strains have sufficiently different growth characteristics and are unlikely to share the same cooperative gene (so that we can safely ignore kin selection [88,113])). This may spatially isolate interacting species [128,129], exert opposing selection pressures on a focal species [62] and result in higher-order interactions [24,130]. Predicting the joint effect of such forces is not straightforward. However, one way to view this issue is to assume that every species adds a dimension to the diagram depicted in figure 3a. Such increased dimensionality may, on the one hand, open up new pathways for individuals to adapt, but, on the other hand, also constrain evolution, because mutations will be increasingly likely to decrease fitness in at least one respect [62,131,132]. This would suggest that evolution will proceed fastest under some intermediate community diversity.
The generation of a conceptual, qualitative model, in which reality is abstracted away to its minimal essence (figure 3a), along with generation of a simple, quantitative model, to see whether our intuitions bear out at least in principle (figure 3c), are useful first steps towards understanding the evolution of microbial communities. However, science is ideally an iterative process of models and experiments, where each informs the other. One way to assess whether our hypotheses can stand the test of reality is to precisely quantify the direct and indirect fitness effects of traits that are thought to drive the interaction between species [133,134]. If a mutation provides a direct benefit, a knock-out mutant will grow more slowly than its wild-type counterpart when grown in monoculture. Conversely, if a mutation provides an indirect benefit, a knock-out mutant will exert a weaker effect (positive or negative) on the growth and/or activity of the interacting species, which may be established using either coculture or growth in spent medium. This indirect effect should then feed back on growth of the focal species. Another approach, which more directly assesses the relative importance of mutations with direct and indirect fitness benefits for evolution, is to perform evolution experiments with multiple microbial species in both spatially structured and well-mixed environments. Careful phenotypic, genotypic and metabolic characterization of evolved isolates can then be used to disentangle mutations with direct and indirect fitness benefits.
While distinguishing direct from indirect fitness effects experimentally is far from trivial, it provides substantial insights into the evolutionary dynamics in microbial communities. In order to understand the long-term fate and the consequences of mutations of community members, it is crucial to understand how selection acts on these mutations, and whether benefits of these mutations to the mutant occur only indirectly, through the interaction with another species. Mutations that have a positive effect on both a focal and a recipient species (such as the cross-feeding interaction) are sometimes automatically assumed to have been selected for the latter effect, even if the most parsimonious explanation might be that these mutations are under direct selection based on the advantage of secreting metabolic by-products. Yet, to make any definite claims about selection history, even in such cases one would need to quantify both direct and indirect effects to ensure that the observed dynamics are consistent with a direct benefit only, and no additional indirect effect needs to be invoked. We, therefore, see great potential in experimental approaches that disentangle direct and indirect fitness effects of mutations.
5. Scaling up
Whether traits typically confer a direct or indirect fitness benefit to the actor can have important consequences. When a mutation in a focal species gets selected for its effect on a second species, be it positive or negative, this second species will now be under stronger selection to acquire a mutation with a similar effect on its partner as well. This is because the indirect benefit that the second species can obtain from an increased investment into the interaction has increased as well. As such we would predict that when evolution proceeds predominantly via the selection of mutations with an indirect fitness benefit, interaction strength will increase over time, potentially to the point of obligate mutualism or bi-stable exclusion. When species initially have an opposite effect on each other (+/−), the long-term direction of this coevolutionary cycle is less clear. However, one possibility is that the outcome depends on which species mutates first. For example, when a first species evolves to excrete more of a compound that inhibits a second species, which has a positive effect on the first species, this second species now will be under stronger selection to decrease its help to the first species, or even to start harming it. Clearly, this prediction will not hold for interactions of which the sign cannot change by definition, such as host–parasite interactions (but note that even such interactions may not be constant [135,136]). Nonetheless, microbial communities are dominated by chemically mediated interactions, suggesting that the selection of indirectly beneficial mutations may occur also in the case of asymmetric interactions.
The overall sign and strength of interspecific interactions have important consequences for properties at the level of the community. More positive interactions are associated with increased productivity, because fewer resources are wasted on competitive traits, and species may engage in division of labour [137,138]. At the same time, an overrepresentation of positive interactions will make a community less stable, because it decreases the number of negative feedback loops, and consequently, when one species is perturbed, it may drag the others down with it to extinction [139]. By contrast, more negative interactions should have a stabilizing effect on the community, because any interaction loop with an odd number of negative connections will result in a negative feedback loop [140]. Negative interactions will also decrease productivity, and whenever interspecific competition becomes stronger than intraspecific competition, this may lead to bi-stable exclusion [141]. More generally, the topology and interaction strengths of any type of network, including the interaction network of a microbial community, are predicted to have far-reaching consequences for the stability and resilience of the system [142–144].
One intriguing question that has received an increasing amount of interest over the past few years is whether microbial communities may be regarded as coherent units, on which selection can act directly [145,146]. If this were the case, we might be able to select for certain desired community-level properties, such as efficiency in producing a specific compound [44]. While this idea has a strong intuitive appeal, experimental results have been mixed [147]. This results from most likely due to the fact that for selection to act on something, a minimum amount of heritability is needed: if a microbial community breaks up into its component parts, or changes in composition owing to ecological and evolutionary forces, sufficiently often relative to it giving birth to a similar community, natural and artificial selection will not be able to act upon the properties of the community.
One way in which such heritability may be increased is if species become so strongly dependent on each other as a result of coevolution that they can no longer live alone [148]. Alternatively, it may result from organisms having coevolved in a community context for sufficiently long that each of them has become locally adapted to its biotic environment, such that foreign species will be unable to replace their resident counterparts [45,149]. Interestingly, our hypotheses predict that both of these types of coevolution should occur significantly more often in spatially structured environments (because it is there that mutations are selected for their indirect fitness effects), which might be an interesting idea to test experimentally. Increased metabolic dependency may also change the spatial association between organisms itself, suggesting that this process may even be subject to a self-reinforcing feedback loop [123].
6. Conclusion
The evolution of microbial communities is a fascinating and complex process that has potentially far-reaching consequences. To understand it, we need an integrated approach that encompasses elements from microbiology, ecology and evolution. Here, we have reviewed the evidence for the importance of the evolution of microbial communities in host-associated as well as free-living communities, and integrated previous empirical and theoretical findings to arrive at a conceptual model of this process. We tested our intuitions using a simple quantitative model and found support for our hypotheses. The next step will be to test these hypotheses experimentally and provide input for a more refined version of the model in turn, thus closing the scientific cycle of models and experiments.
7. Methods
In our cellular automaton model, we simulated the cell division and death dynamics of two species, A and B, on a 40 × 40 grid with periodic boundary conditions, mimicking a spatially structured environment. The A species also has a mutant variant A′. The fitness of each cell i, which determines the probability it will divide at the next time step, is the sum of a basal fitness r and a term proportional to the total fitness of all cells of opposite species in its immediate eight-cell neighbourhood, weighed by an interaction parameter (v):
where the basal fitness parameters are and , while the interaction parameters are and . That is, the wild-type A and B cells have identical basal fitness and help each other equally, while the mutant A′ provides a stronger benefit to B but at a cost to its basal fitness.
We start with a grid that is fully occupied by cells randomly chosen to be either A or B, each with initial fitness randomly drawn from a uniform distribution. In each time step, we start by updating the fitness of all cells according to the above equations, constraining growth rates between 0 and 1. Then we randomly choose one cell in the grid to reproduce with probability that is proportional to its updated fitness, placing the new cell in one of the eight neighbouring positions. Next, we randomly choose another cell on the grid to die, and the new cell pushes all cells in the direction in which it is placed relative to its mother cell towards the now-empty spot, taking a path with only a single turn. We define one population turnover as 40 × 40 = 1600 of such time steps.
We started by running this simulation for 10 population turnovers to let the population reach an approximately steady-state configuration. Then we introduced a single A′ mutant and ran the simulation until either the mutant went extinct or reached an ‘establishment’ threshold, which we defined as 10 cells; we chose this threshold to be twice the maximum number of mutant cells that we observed for a mutant destined for extinction (based on 20 preliminary trials). We repeated this procedure 100 times, all starting from the same initial population. Altogether the mutant established in 41 out of 100 of these simulations.
As a control, we performed the same simulations but let the fitness of each cell depend on interactions from all other cells in the population (rather than just cells in the immediate neighbourhood), thus mimicking a well-mixed environment:
where we rescale the interactions by 200 to keep parameters comparable between the two models, since in the well-mixed case each cell has approximately 200 times more neighbours than in the spatially structured case.
We chose 16 cells as the establishment threshold in this case, since eight cells were the maximum number reached by mutants destined for extinction (based on 20 preliminary trials). Based on this criterion, we observed establishment in 8 out of 100 simulations. Because we suspected that these putative establishments might actually be due to genetic drift rather than positive selection, we continued these simulations for another 50 population turnovers. Six of these cases indeed led to extinction during that time, while in the remaining two cases, the mutant was still present at low, fluctuating frequencies (less than 4%) by the end and did not substantially affect the frequency of the wild-type A.
We plotted the first 10 replicates of each simulation for figure 3c. We ran all simulations in R.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
F.A.G. was supported by an ETH Fellowship and a European Molecular Biology Organization (EMBO) Long-Term Postdoctoral Fellowship (ALTF 335-2018); M.M. was supported by an Ambizione Fellowship from the Swiss National Science Foundation (PZ00P3_180147); F.A.G. and M.A. were supported by the Simons Foundation: The Simons Collaboration on Principles of Microbial Ecosystems (PriME no. 542389). M.A. was supported by a grant from the Swiss National Science Foundation (31003A_169978).
References
- 1.Strom SL. 2008. Microbial ecology of ocean biogeochemistry: a community perspective. Science 320, 1043–1045. ( 10.1126/science.1153527) [DOI] [PubMed] [Google Scholar]
- 2.Berendsen RL, Pieterse CMJ, Bakker PAHM. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486. ( 10.1016/j.tplants.2012.04.001) [DOI] [PubMed] [Google Scholar]
- 3.Maukonen J, Saarela M. 2009. Microbial communities in industrial environment. Curr. Opin. Microbiol. 12, 238–243. ( 10.1016/j.mib.2009.04.002) [DOI] [PubMed] [Google Scholar]
- 4.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. 2018. Current understanding of the human microbiome. Nat. Med. 24, 392–400. ( 10.1038/nm.4517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fierer N. 2017. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15, 579–590. ( 10.1038/nrmicro.2017.87) [DOI] [PubMed] [Google Scholar]
- 6.Whitman WB, Coleman DC, Wiebe WJ. 1998. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583. ( 10.1073/pnas.95.12.6578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widder S, et al. 2016. Challenges in microbial ecology: building predictive understanding of community function and dynamics. ISME J. 10, 2557–2568. ( 10.1038/ismej.2016.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zomorrodi AR, Segrè D. 2016. Synthetic ecology of microbes: mathematical models and applications. J. Mol. Biol. 428, 837–861. ( 10.1016/j.jmb.2015.10.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villani F, Pepe O, Mauriello G, Salzano G, Moschetti G, Coppola S. 1995. Antilisterial activity of thermophilin 347, a bacteriocin produced by Streptococcus thermophilus. Int. J. Food Microbiol. 25, 179–190. ( 10.1016/0168-1605(94)00153-W) [DOI] [PubMed] [Google Scholar]
- 10.Sieuwerts S, De Bok FAM, Hugenholtz J, Van Hylckama Vlieg JET. 2008. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol. 74, 4997–5007. ( 10.1128/AEM.00113-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. 2010. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. ( 10.1038/nrmicro2259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basler M, Ho BT, Mekalanos JJ. 2013. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152, 884–894. ( 10.1016/j.cell.2013.01.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadell CD, Drescher K, Foster KR. 2016. Spatial structure, cooperation and competition in biofilms. Nat. Rev. Microbiol. 14, 589–600. ( 10.1038/nrmicro.2016.84) [DOI] [PubMed] [Google Scholar]
- 14.Estrela S, Libby E, Van Cleve J, Débarre F, Deforet M, Harcombe WR, Peña J, Brown SP, Hochberg ME. 2019. Environmentally mediated social dilemmas. Trends Ecol. Evol. 34, 6–18. ( 10.1016/j.tree.2018.10.004) [DOI] [PubMed] [Google Scholar]
- 15.Riley MA, Gordon DM. 1999. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 7, 129–133. ( 10.1016/S0966-842X(99)01459-6) [DOI] [PubMed] [Google Scholar]
- 16.Dolinšek J, Goldschmidt F, Johnson DR. 2016. Synthetic microbial ecology and the dynamic interplay between microbial genotypes. FEMS Microbiol. Rev. 40, 961–979. ( 10.1093/femsre/fuw024) [DOI] [PubMed] [Google Scholar]
- 17.Foster KR, Bell T. 2012. Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 22, 1845–1850. ( 10.1016/j.cub.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 18.Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C. 2013. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37, 384–406. ( 10.1111/1574-6976.12019) [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan S, Kaplan CN, Mahadevan L. 2019. A multiphase theory for spreading microbial swarms and films. eLife 8, e42697 ( 10.7554/eLife.42697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Corral R, Liu J, Prindle A, Süel GM, Garcia-Ojalvo J. 2019. Metabolic basis of brain-like electrical signalling in bacterial communities. Phil. Trans. R. Soc. B 374, 20180382 ( 10.1098/rstb.2018.0382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratzke C, Gore J. 2018. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 16, e2004248 ( 10.1371/journal.pbio.2004248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enke TN, Datta MS, Schwartzman J, Cermak N, Schmitz D, Barrere J, Pascual-García A, Cordero OX. 2019. Modular assembly of polysaccharide-degrading marine microbial communities. Curr. Biol. 29, 1528–1535. ( 10.1016/j.cub.2019.03.047) [DOI] [PubMed] [Google Scholar]
- 23.Goldford JE, Lu N, Bajić D, Estrela S, Tikhonov M, Sanchez-Gorostiaga A, Segrè D, Mehta P, Sanchez A. 2018. Emergent simplicity in microbial community assembly. Science 361, 469–474. ( 10.1126/science.aat1168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman J, Higgins LM, Gore J. 2017. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 1, 0109 ( 10.1038/s41559-017-0109) [DOI] [PubMed] [Google Scholar]
- 25.Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. 2016. Within-host evolution of bacterial pathogens. Nat. Rev. Microbiol. 14, 150–162. ( 10.1038/nrmicro.2015.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman TD, Flett KB, Yelin I, Martin TR, McAdam AJ, Priebe GP, Kishony R. 2014. Genetic variation of a bacterial pathogen within individuals with cystic fibrosis provides a record of selective pressures. Nat. Genet. 46, 82–87. ( 10.1038/ng.2848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao S, Lieberman TD, Poyet M, Kauffman KM, Gibbons SM, Groussin M, Xavier RJ, Alm EJ. 2019. Adaptive evolution within gut microbiomes of healthy people. Cell Host Microbe 25, 656–667. ( 10.1016/j.chom.2019.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sousa A, Frazão N, Ramiro RS, Gordo I. 2017. Evolution of commensal bacteria in the intestinal tract of mice. Curr. Opin. Microbiol. 38, 114–121. ( 10.1016/j.mib.2017.05.007) [DOI] [PubMed] [Google Scholar]
- 29.Barroso-Batista J, Sousa A, Lourenço M, Bergman ML, Sobral D, Demengeot J, Xavier KB, Gordo I. 2014. The first steps of adaptation of Escherichia coli to the gut are dominated by soft sweeps. PLoS Genet. 10, e1004182 ( 10.1371/journal.pgen.1004182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garud NR, Good BH, Hallatschek O, Pollard KS. 2019. Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol. 17, e3000102 ( 10.1371/journal.pbio.3000102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denef VJ, Banfield JF. 2012. In situ evolutionary rate measurements show ecological success of recently emerged bacterial hybrids. Science 336, 462–466. ( 10.1126/science.1218389) [DOI] [PubMed] [Google Scholar]
- 32.Bendall ML, et al. 2016. Genome-wide selective sweeps and gene-specific sweeps in natural bacterial populations. ISME J. 10, 1589–1601. ( 10.1038/ismej.2015.241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemme CL, et al. 2016. Lateral gene transfer in a heavy metal-contaminated-groundwater microbial community. mBio 7, e02234-15 ( 10.1128/mBio.02234-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su JQ, An XL, Li B, Chen QL, Gillings MR, Chen H, Zhang T, Zhu YG. 2017. Metagenomics of urban sewage identifies an extensively shared antibiotic resistome in China. Microbiome 5, 84 ( 10.1186/s40168-017-0298-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Mering C, Hugenholtz P, Raes J, Tringe SG, Doerks T, Jensen LJ, Ward N, Bork P. 2007. Quantitative phylogenetic assessment of microbial communities in diverse environments. Science 315, 1126–1130. ( 10.1126/science.1133420) [DOI] [PubMed] [Google Scholar]
- 36.Starnawski P, et al. 2017. Microbial community assembly and evolution in subseafloor sediment. Proc. Natl Acad. Sci. USA 114, 2940–2945. ( 10.1073/pnas.1614190114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li SJ, et al. 2014. Microbial communities evolve faster in extreme environments. Scient. Rep. 4, 6205 ( 10.1038/srep06205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swenson W, Wilson DS, Elias R. 2000. Artificial ecosystem selection. Proc. Natl Acad. Sci. USA 97, 9110–9114. ( 10.1073/pnas.150237597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blouin M, Karimi B, Mathieu J, Lerch TZ. 2015. Levels and limits in artificial selection of communities. Ecol. Lett. 18, 1040–1048. ( 10.1111/ele.12486) [DOI] [PubMed] [Google Scholar]
- 40.Wright RJ, Gibson MI, Christie-Oleza JA. 2019. Understanding microbial community dynamics to improve optimal microbiome selection. Microbiome 7, 85 ( 10.1186/s40168-019-0702-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barraclough TG. 2015. How do species interactions affect evolutionary dynamics across whole communities? Annu. Rev. Ecol. Evol. Syst. 46, 25–48. ( 10.1146/annurev-ecolsys-112414-054030) [DOI] [Google Scholar]
- 42.Low-Décarie E, Kolber M, Homme P, Lofano A, Dumbrell A, Gonzalez A, Bell G. 2015. Community rescue in experimental metacommunities. Proc. Natl Acad. Sci. USA 112, 14 307–14 312. ( 10.1073/pnas.1513125112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gómez P, Paterson S, De Meester L, Liu X, Lenzi L, Sharma MD, McElroy K, Buckling A. 2016. Local adaptation of a bacterium is as important as its presence in structuring a natural microbial community. Nat. Commun. 7, 12453 ( 10.1038/ncomms12453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie L, Yuan AE, Shou W. 2019. Simulations reveal challenges to artificial community selection and possible strategies for success. PLoS Biol. 17, e3000295 ( 10.1371/journal.pbio.3000295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tikhonov M. 2016. Community-level cohesion without cooperation. eLife 5, e15747 ( 10.7554/eLife.15747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierocinski P, et al. 2017. A single community dominates structure and function of a mixture of multiple methanogenic communities. Curr. Biol. 27, 3390–3395. ( 10.1016/j.cub.2017.09.056) [DOI] [PubMed] [Google Scholar]
- 47.Ereshefsky M, Pedroso M. 2015. Rethinking evolutionary individuality. Proc. Natl Acad. Sci. USA 112, 10 126–10 132. ( 10.1073/pnas.1421377112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke E. 2016. Levels of selection in biofilms: multispecies biofilms are not evolutionary individuals. Biol. Philos. 31, 191–212. ( 10.1007/s10539-016-9517-3) [DOI] [Google Scholar]
- 49.Elena SF, Lenski RE. 2003. Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4, 457–469. ( 10.1038/nrg1088) [DOI] [PubMed] [Google Scholar]
- 50.Barrick JE, Lenski RE. 2013. Genome dynamics during experimental evolution. Nat. Rev. Genet. 14, 827–839. ( 10.1038/nrg3564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brockhurst MA, Koskella B. 2013. Experimental coevolution of species interactions. Trends Ecol. Evol. 28, 367–375. ( 10.1016/j.tree.2013.02.009) [DOI] [PubMed] [Google Scholar]
- 52.Hall AR, Scanlan PD, Morgan AD, Buckling A. 2011. Host–parasite coevolutionary arms races give way to fluctuating selection. Ecol. Lett. 14, 635–642. ( 10.1111/j.1461-0248.2011.01624.x) [DOI] [PubMed] [Google Scholar]
- 53.Friman VP, Hiltunen T, Laakso J, Kaitala V. 2008. Availability of prey resources drives evolution of predator–prey interaction. Proc. R. Soc. B 275, 1625–1633. ( 10.1098/rspb.2008.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillesland KL, Stahl DA. 2010. Rapid evolution of stability and productivity at the origin of a microbial mutualism. Proc. Natl Acad. Sci. USA 107, 2124–2129. ( 10.1073/pnas.0908456107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harcombe WR, Chacón JM, Adamowicz EM, Chubiz LM, Marx CJ. 2018. Evolution of bidirectional costly mutualism from byproduct consumption. Proc. Natl Acad. Sci. USA 115, 12 000–12 004. ( 10.1073/pnas.1810949115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen SK, Rainey PB, Haagensen JAJ, Molin S. 2007. Evolution of species interactions in a biofilm community. Nature 445, 533–536. ( 10.1038/nature05514) [DOI] [PubMed] [Google Scholar]
- 57.Andrade-Domínguez A, Salazar E, Del Carmen Vargas-Lagunas M, Kolter R, Encarnación S. 2014. Eco-evolutionary feedbacks drive species interactions. ISME J. 8, 1041–1054. ( 10.1038/ismej.2013.208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celiker H, Gore J. 2014. Clustering in community structure across replicate ecosystems following a long-term bacterial evolution experiment. Nat. Commun. 5, 4643 ( 10.1038/ncomms5643) [DOI] [PubMed] [Google Scholar]
- 59.Gómez P, Buckling A. 2011. Bacteria-phage antagonistic coevolution in soil. Science 332, 106–109. ( 10.1126/science.1198767) [DOI] [PubMed] [Google Scholar]
- 60.Jousset A, Eisenhauer N, Merker M, Mouquet N, Scheu S. 2016. High functional diversity stimulates diversification in experimental microbial communities. Sci. Adv. 2, e1600124 ( 10.1126/sciadv.1600124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall JPJ, Harrison E, Brockhurst MA. 2018. Competitive species interactions constrain abiotic adaptation in a bacterial soil community. Evol. Lett. 2, 580–589. ( 10.1002/evl3.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClean D, Friman V, Finn A, Salzberg LI, Donohue I. 2019. Coping with multiple enemies: pairwise interactions do not predict evolutionary change in complex multitrophic communities. Oikos 128, 1588–1599. ( 10.1111/oik.06586) [DOI] [Google Scholar]
- 63.Lawrence D, Fiegna F, Behrends V, Bundy JG, Phillimore AB, Bell T, Barraclough TG. 2012. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 ( 10.1371/journal.pbio.1001330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiegna F, Moreno-Letelier A, Bell T, Barraclough TG. 2015. Evolution of species interactions determines microbial community productivity in new environments. ISME J. 9, 1235–1245. ( 10.1038/ismej.2014.215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Meester L, et al. 2019. Analysing eco-evolutionary dynamics—the challenging complexity of the real world. Funct. Ecol. 33, 43–59. ( 10.1111/1365-2435.13261) [DOI] [Google Scholar]
- 66.Schoener TW. 2011. The newest synthesis: understanding ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 67.Bell G. 2013. Evolutionary rescue and the limits of adaptation. Phil. Trans. R. Soc. B 368, 20120080 ( 10.1098/rstb.2012.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips R. 2015. Theory in biology: figure 1 or figure 7? Trends Cell Biol. 25, 723–729. ( 10.1016/j.tcb.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaccaria M, Dedrick S, Momeni B. 2017. Modeling microbial communities: a call for collaboration between experimentalists and theorists. Processes 5, 53 ( 10.3390/pr5040053) [DOI] [Google Scholar]
- 70.Crow JMK. 1970. An introduction to population genetics theory. New York, NY: Harper and Row. [Google Scholar]
- 71.Orr HA. 2009. Fitness and its role in evolutionary genetics. Nat. Rev. Genet. 10, 531–539. ( 10.1038/nrg2603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Visser JAGM, Rozen DE. 2006. Clonal interference and the periodic selection of new beneficial mutations in Escherichia coli. Genetics 172, 2093–2100. ( 10.1534/genetics.105.052373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neher RA, Shraiman BI, Fisher DS. 2010. Rate of adaptation in large sexual populations. Genetics 184, 467–481. ( 10.1534/genetics.109.109009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Korolev KS, Müller MJI, Karahan N, Murray AW, Hallatschek O, Nelson DR. 2012. Selective sweeps in growing microbial colonies. Phys. Biol. 9, 026008 ( 10.1088/1478-3975/9/2/026008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parsons TL, Quince C, Plotkin JB. 2010. Some consequences of demographic stochasticity in population genetics. Genetics 185, 1345–1354. ( 10.1534/genetics.110.115030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative characters. Sunderland, MA: Sinauer. [Google Scholar]
- 77.Damore JA, Gore J. 2012. Understanding microbial cooperation. J. Theor. Biol. 299, 31–41. ( 10.1016/j.jtbi.2011.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gardner A. 2008. The Price equation. Curr. Biol. 18, R198–R202. ( 10.1016/j.cub.2008.01.005) [DOI] [PubMed] [Google Scholar]
- 79.Otto S, Day T. 2007. A biologist‘s guide to mathematical modelling in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 80.MacArthur R. 1970. Species packing and competitive equilibrium for many species. Theor. Popul. Biol. 1, 1–11. ( 10.1016/0040-5809(70)90039-0) [DOI] [PubMed] [Google Scholar]
- 81.Momeni B, Xie L, Shou W. 2017. Lotka-Volterra pairwise modeling fails to capture diverse pairwise microbial interactions. eLife 6, 25051 ( 10.7554/elife.25051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orth JD, Thiele I, Palsson BØ. 2011. What is flux balance analysis? Nat. Biotechnol. 28, 245–248. ( 10.1038/nbt.1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harcombe WR, et al. 2014. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 7, 1104–1115. ( 10.1016/j.celrep.2014.03.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bar-On YM, Milo R. 2019. Towards a quantitative view of the global ubiquity of biofilms. Nat. Rev. Microbiol. 17, 199–200. ( 10.1038/s41579-019-0162-0) [DOI] [PubMed] [Google Scholar]
- 85.Dal Co A, van Vliet S, Kiviet DJ, Schlegel S, Ackermann M.2019. Short-range interactions govern cellular dynamics in microbial multi-genotype systems. bioRxiv, 530584. (doi:10.1101/530584)
- 86.Hellweger FL, Clegg RJ, Clark JR, Plugge CM, Kreft J-U. 2016. Advancing microbial sciences by individual-based modelling. Nat. Rev. Microbiol. 2, 2–4. ( 10.1038/nrmicro.2016.62) [DOI] [PubMed] [Google Scholar]
- 87.Ferrer J, Prats C, López D. 2008. Individual-based modelling: an essential tool for microbiology. J. Biol. Phys. 34, 19–37. ( 10.1007/s10867-008-9082-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 89.Frickel J, Sieber M, Becks L. 2016. Eco-evolutionary dynamics in a coevolving host–virus system. Ecol. Lett. 19, 450–459. ( 10.1111/ele.12580) [DOI] [PubMed] [Google Scholar]
- 90.Hendry AP. 2019. A critique for eco-evolutionary dynamics. Funct. Ecol. 33, 84–94. ( 10.1111/1365-2435.13244) [DOI] [Google Scholar]
- 91.Smith JM, Price GR. 1973. The logic of animal conflict. Nature 246, 15–18. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 92.Frey E. 2010. Evolutionary game theory: theoretical concepts and applications to microbial communities. Phys. A Stat. Mech. Appl. 389, 4265–4298. ( 10.1016/j.physa.2010.02.047) [DOI] [Google Scholar]
- 93.Nowak MA, Tarnita CE, Antal T. 2010. Evolutionary dynamics in structured populations. Phil. Trans. R. Soc. B 365, 19–30. ( 10.1098/rstb.2009.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conlin PL, Chandler JR, Kerr B. 2014. Games of life and death: antibiotic resistance and production through the lens of evolutionary game theory. Curr. Opin. Microbiol. 21, 35–44. ( 10.1016/j.mib.2014.09.004) [DOI] [PubMed] [Google Scholar]
- 95.Page KM, Nowak MA. 2002. Unifying evolutionary dynamics. J. Theor. Biol. 219, 93–98. ( 10.1016/S0022-5193(02)93112-7) [DOI] [PubMed] [Google Scholar]
- 96.Gore J, Youk H, Van Oudenaarden A. 2009. Snowdrift game dynamics and facultative cheating in yeast. Nature 459, 253–256. ( 10.1038/nature07921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Czárán TL, Hoekstra RF. 2003. Killer-sensitive coexistence in metapopulations of micro-organisms. Proc. R. Soc. Lond. B 270, 1373–1378. ( 10.1098/rspb.2003.2338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kerr B, Riley MA, Feldman MW, Bohannan BJM. 2002. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature 418, 171–174. ( 10.1038/nature00823) [DOI] [PubMed] [Google Scholar]
- 99.Frank SA. 2010. Microbial secretor–cheater dynamics. Phil. Trans. R. Soc. B 365, 2515–2522. ( 10.1098/rstb.2010.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doebeli M, Knowlton N. 1998. The evolution of interspecific mutualisms. Proc. Natl Acad. Sci. USA 95, 8676–8680. ( 10.1073/pnas.95.15.8676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morozov A, Best A. 2012. Predation on infected host promotes evolutionary branching of virulence and pathogens' biodiversity. J. Theor. Biol. 307, 29–36. ( 10.1016/j.jtbi.2012.04.023) [DOI] [PubMed] [Google Scholar]
- 102.Friedman J, Gore J. 2017. Ecological systems biology: the dynamics of interacting populations. Curr. Opin. Syst. Biol. 1, 114–121. ( 10.1016/j.coisb.2016.12.001) [DOI] [Google Scholar]
- 103.Gardner A, West SA, Buckling A. 2004. Bacteriocins, spite and virulence. Proc. R. Soc. Lond. B 271, 1529–1535. ( 10.1098/rspb.2004.2756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fenton A, Antonovics J, Brockhurst MA. 2009. Inverse-gene-for-gene infection genetics and coevolutionary dynamics. Am. Nat. 174, E230–E242. ( 10.1086/645087) [DOI] [PubMed] [Google Scholar]
- 105.Sasaki A. 2000. Host–parasite coevolution in a multilocus gene-for-gene system. Proc. R. Soc. Lond. B 267, 2183–2188. ( 10.1098/rspb.2000.1267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Durrett R, Mayberry J. 2010. Evolution in predator–prey systems. Stoch. Process. Appl. 120, 1364–1392. ( 10.1016/j.spa.2010.03.011) [DOI] [Google Scholar]
- 107.Nuismer SL, Doebeli M, Browning D. 2005. The coevolutionary dynamics of antagonistic interactions mediated by quantitative traits with evolving variances. Evolution 59, 2073–2082. ( 10.1111/j.0014-3820.2005.tb00918.x) [DOI] [PubMed] [Google Scholar]
- 108.Estrela S, Trisos CH, Brown SP. 2012. From metabolism to ecology: cross-feeding interactions shape the balance between polymicrobial conflict and mutualism. Am. Nat. 180, 566–576. ( 10.1086/667887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rivett DW, Scheuerl T, Culbert CT, Mombrikotb SB, Johnstone E, Barraclough TG, Bell T. 2016. Resource-dependent attenuation of species interactions during bacterial succession. ISME J. 10, 2259–2268. ( 10.1038/ismej.2016.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodríguez-Verdugo A, Vulin C, Ackermann M. 2019. The rate of environmental fluctuations shapes ecological dynamics in a two-species microbial system. Ecol. Lett. 22, 838–846. ( 10.1111/ele.13241) [DOI] [PubMed] [Google Scholar]
- 111.West SA, Griffin AS, Gardner A. 2007. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432. ( 10.1111/j.1420-9101.2006.01258.x) [DOI] [PubMed] [Google Scholar]
- 112.Mitri S, Foster KR. 2013. The genotypic view of social interactions in microbial communities. Annu. Rev. Genet. 47, 247–273. ( 10.1146/annurev-genet-111212-133307) [DOI] [PubMed] [Google Scholar]
- 113.Hamilton WD. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 114.Axelrod R, Hamilton W. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 115.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160. ( 10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 116.Fletcher JA, Doebeli M. 2009. A simple and general explanation for the evolution of altruism. Proc. R. Soc. B 276, 13–19. ( 10.1098/rspb.2008.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harcombe W. 2010. Novel cooperation experimentally evolved between species. Evolution 64, 2166–2172. ( 10.1111/j.1558-5646.2010.00959.x) [DOI] [PubMed] [Google Scholar]
- 118.Werner GDA, et al. 2014. Evolution of microbial markets. Proc. Natl Acad. Sci. USA 111, 1237–1244. ( 10.1073/pnas.1315980111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nowak MA. 2006. Five rules for the evolution of cooperation. Science 314, 1560–1563. ( 10.1126/science.1133755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brosnan SF, De Waal FBM. 2002. A proximate perspective on reciprocal altruism. Hum. Nat. 13, 129–152. ( 10.1007/s12110-002-1017-2) [DOI] [PubMed] [Google Scholar]
- 121.Bull JJ, Rice WR. 1991. Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 149, 63–74. ( 10.1016/S0022-5193(05)80072-4) [DOI] [PubMed] [Google Scholar]
- 122.Pande S, Kaftan F, Lang S, Svato A, Germerodt S, Kost C. 2016. Privatization of cooperative benefits stabilizes mutualistic cross-feeding interactions in spatially structured environments. ISME J. 10, 1413–1423. ( 10.1038/ismej.2015.212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marchal M, Goldschmidt F, Derksen-Müller SN, Panke S, Ackermann M, Johnson DR. 2017. A passive mutualistic interaction promotes the evolution of spatial structure within microbial populations. BMC Evol. Biol. 17, 106 ( 10.1186/s12862-017-0950-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamamura N, Higashi M, Behera N, Wakano JY. 2004. Evolution of mutualism through spatial effects. J. Theor. Biol. 226, 421–428. ( 10.1016/j.jtbi.2003.09.016) [DOI] [PubMed] [Google Scholar]
- 125.Stump SM, Johnson EC, Sun Z, Klausmeier CA. 2018. How spatial structure and neighbor uncertainty promote mutualists and weaken black queen effects. J. Theor. Biol. 446, 33–60. ( 10.1016/j.jtbi.2018.02.031) [DOI] [PubMed] [Google Scholar]
- 126.Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M. 2009. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 12, 13–21. ( 10.1111/j.1461-0248.2008.01254.x) [DOI] [PubMed] [Google Scholar]
- 127.Débarre F, Hauert C, Doebeli M. 2014. Social evolution in structured populations. Nat. Commun. 5, 3409 ( 10.1038/ncomms4409) [DOI] [PubMed] [Google Scholar]
- 128.Momeni B, Brileya KA, Fields MW, Shou W. 2013. Strong inter-population cooperation leads to partner intermixing in microbial communities. eLife 2, e00230 ( 10.7554/eLife.00230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mitri S, Xavier JB, Foster KR. 2011. Social evolution in multispecies biofilms. Proc. Natl Acad. Sci. USA 108, 10 839–10 846. ( 10.1073/pnas.1100292108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Senay Y, John G, Knutie SA, Ogbunugafor CB.2019. Deconstructing higher-order interactions in the microbiota: a theoretical examination. bioRxiv, 647156. (doi:10.1101/647156)
- 131.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 132.Orr HA. 2005. The genetic theory of adaptation: a brief history. Nat. Rev. Genet. 6, 119–127. ( 10.1038/nrg1523) [DOI] [PubMed] [Google Scholar]
- 133.Rakoff-Nahoum S, Foster KR, Comstock LE. 2016. The evolution of cooperation within the gut microbiota. Nature 533, 255–259. ( 10.1038/nature17626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rainey PB, Desprat N, Driscoll WW, Zhang XX. 2014. Microbes are not bound by sociobiology: response to Kümmerli and Ross-Gillespie (2013). Evolution 68, 3344–3355. ( 10.1111/evo.12508) [DOI] [PubMed] [Google Scholar]
- 135.Shapiro JW, Turner PE. 2018. Evolution of mutualism from parasitism in experimental virus populations. Evolution 72, 707–712. ( 10.1111/evo.13440) [DOI] [PubMed] [Google Scholar]
- 136.Harrison E, Guymer D, Spiers AJ, Paterson S, Brockhurst MA. 2015. Parallel compensatory evolution stabilizes plasmids across the parasitism-mutualism continuum. Curr. Biol. 25, 2034–2039. ( 10.1016/j.cub.2015.06.024) [DOI] [PubMed] [Google Scholar]
- 137.Lindemann SR, Bernstein HC, Song HS, Fredrickson JK, Fields MW, Shou W, Johnson DR, Beliaev AS. 2016. Engineering microbial consortia for controllable outputs. ISME J. 10, 2077–2084. ( 10.1038/ismej.2016.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lilja EE, Johnson DR. 2016. Segregating metabolic processes into different microbial cells accelerates the consumption of inhibitory substrates. ISME J. 10, 1568–1578. ( 10.1038/ismej.2015.243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coyte KZ, Schluter J, Foster KR. 2015. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666. ( 10.1126/science.aad2602) [DOI] [PubMed] [Google Scholar]
- 140.Kéfi S, Holmgren M, Scheffer M. 2016. When can positive interactions cause alternative stable states in ecosystems? Funct. Ecol. 30, 88–97. ( 10.1111/1365-2435.12601) [DOI] [Google Scholar]
- 141.Wright ES, Vetsigian KH. 2016. Inhibitory interactions promote frequent bistability among competing bacteria. Nat. Commun. 7, 11274 ( 10.1038/ncomms11274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.May RM. 1972. Will a large complex system be stable? Nature 238, 413–414. ( 10.1038/238413a0) [DOI] [PubMed] [Google Scholar]
- 143.Allesina S, Tang S. 2012. Stability criteria for complex ecosystems. Nature 483, 205–208. ( 10.1038/nature10832) [DOI] [PubMed] [Google Scholar]
- 144.Scheffer M, et al. 2012. Anticipating critical transitions. Science 338, 344–348. ( 10.1126/science.1225244) [DOI] [PubMed] [Google Scholar]
- 145.Day MD, Beck D, Foster JA. 2011. Microbial communities as experimental units. Bioscience 61, 398–406. ( 10.1525/bio.2011.61.5.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liautaud K, van Nes EH, Barbier M, Scheffer M, Loreau M. 2019. Superorganisms or loose collections of species? A unifying theory of community patterns along environmental gradients. Ecol. Lett. 22, 1243–1252. ( 10.1111/ele.13289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Escalante AE, Rebolleda-Gómez M, Benítez M, Travisano M. 2015. Ecological perspectives on synthetic biology: insights from microbial population biology. Front. Microbiol. 6, 143 ( 10.3389/fmicb.2015.00143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morris JJ, Lenski RE, Zinser ER. 2012. The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio 3, e00036-12 ( 10.1128/mBio.00036-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.