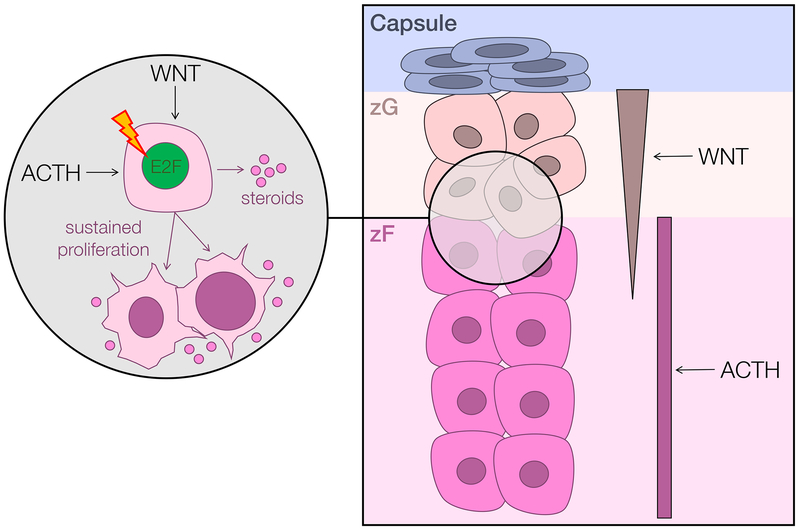
Figure 2. Murine models of adrenocortical homeostasis and cancer point to a putative cell of origin for Wnt-active ACC.
Schematized right is a portion of the capsular/cortical unit of the adrenal cortex. The upper layer of the adrenal cortex, the zona glomerulosa (zG), bears a gradient of active Wnt signaling and produces mineralocorticoids. The second layer of the adrenal cortex, the zona fasciculata (zF), proliferates and produces glucocorticoids in response to ACTH/PKA. Comprehensive molecular profiling studies identified recurrent mutations leading to constitutive activation of both ACTH/PKA and Wnt signaling ACC (7,8), and demonstrated that Wnt/β-catenin pathway alterations are significantly associated with clinical cortisol production (8). Recent mouse models of ACTH-driven zF regeneration (43), augmented Wnt/β-catenin signaling supported by ZNRF3 deficiency (44), and sustained proliferation triggered by adrenocortical expression of the SV40 large T-antigen (45) also demonstrate a unique interplay between Wnt/β-catenin and ACTH/PKA signaling in enabling proliferation of cells residing in the zG/zF boundary. Taken together, these studies support the existence of a small population of zG/zF boundary cells that are capable of rapidly proliferating in response to sustained Wnt/β-catenin and/or ACTH signaling. Prolonged cell cycle activation (schematized here by E2F) may render these cells susceptible to malignant transformation and ligand-independent growth.