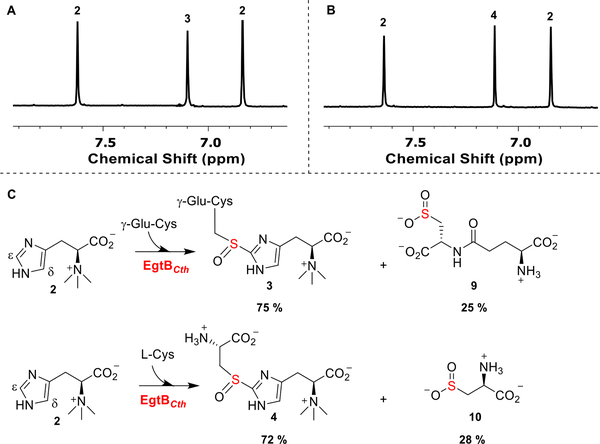
Figure 1.
1H NMR analysis of EgtBCth reactions. (A) EgtBCth reaction under EgtB reaction conditions. The two hydrogens of compound 2 imidazole side chain are labeled as 2, and the hydrogen of compound 3 imidazole hydrogen is labeled as 3. (B) EgtBCth reaction under Egt1-conditions. Compound 4 imidazole hydrogen is labeled 4. (C) Ratios of sulfoxide synthase and cysteine dioxygenase activity of EgtBCth under EgtB- and Egt1-type of reaction conditions. These two competing pathways are present in all three sulfoxide synthases (Egt1, EgtB, and OvoA). Notably, a significant level of cysteine dioxygenase activity was observed in EgtBCth-catalysis.