Abstract
Gastroenteritis of viral origin has emerged as a major cause of morbidity and mortality in dogs during the last two decades. Amongst the viral etiologies responsible for gastroenteritis in dogs, canine parvovirus (CPV) is considered as the most pathogenic. The disease is characterized by hemorrhagic enteritis, bloody diarrhoea and myocarditis in young pups. The present study was carried out to examine alterations in oxidative stress indices in the erythrocytes from dogs suffering from gastroenteritis with or without canine parvoviral infection as confirmed by CPV-DNA amplification from faeces using specific primers for CPV-2 as well as CPV-2a and CPV-2b variants by polymerase chain reaction (PCR). The present investigation utilized clinical cases of dogs with signs of acute diarrhea (n = 56), and 14 more apparently healthy dogs of similar age group. Erythrocytic oxidative stress indices such as lipid peroxides level and antioxidant enzymes like superoxide dismutase and catalase activity, and blood micro-mineral (iron, copper, cobalt and zinc) status were analyzed in each dog (n = 70). The acute cases of gastroenteritis in dogs were associated with altered erythrocytic lipid peroxidation as evident by estimation of malonaldehyde (MDA) concentration. The activities of antioxidant enzymes catalase and superoxide dismutase, the first line of antioxidant defense against damaging effects of free radicals, were also altered. The alterations in oxidative stress indices were more pronounced in cases with involvement of canine parvovirus as compared to parvo-negative cases. Our results also revealed decreased blood zinc level in diarrhoea in dogs irrespective of involvement of canine parvovirus.
Keywords: Oxidative stress, Blood micro-mineral, Gastroenteritis, CPV, Dogs
1. Introduction
During last three decades, parvovirus infection has emerged as a major cause of gastroenteritis in dogs. The infection has been reported from many parts of the world and it continues as a serious threat to canine population despite the presence of vaccine (Carmichael, 2005). Symptomatic treatment is resorted to in case of parvoviral disease in dogs to reduce mortality in the absence of efficacious antiviral therapies for very specific treatment. Therapeutic efficacy of a recombinant feline interferon (type omega) preparation has also been evaluated, and such treatment provided better therapeutic results (Martin et al., 2002, de Mari et al., 2003).
Oxidative stress has been implicated in the pathogenesis in many diseases and inflammatory conditions. It occurs when redox homeostasis within the cell is altered due to either an over production of reactive oxygen species (ROS) or deficiency of counteracting antioxidant system. The pathogenesis of several viral diseases like Acquired Immunodeficiency Syndrome (AIDS), influenza and hepatitis in humans has been linked to oxidative stress (Semba and Tang, 1999), and significantly higher level of lipid peroxides, reduced vitamin-C, serum selenium have been reported in HIV positive patients (Allard et al., 1998). Oxidative stress was also reported to be involved in pathogenesis of several infections in dogs such as Babesia gibsoni, (Murase et al., 1996), Leishmaniasis (Bildik et al., 2004), and in concomitant infection with Babesis and Ehrlichia (Kumar et al., 2006). However, there seems to have no report on possible involvement of oxidative stress in gastroenteritis in dogs with or without canine parvoviral infection.
Reactive oxygen species (ROS) are produced as a defense mechanism by neutrophils and other cells during immune activation by viruses for amplification of signals (Peterhans et al., 1987). Lipid peroxidation resulting from attacks of generated reactive oxygen species on double bonds in poly unsaturated fatty acids (PUFA) reduces the cellular elasticity and enhances cellular damage (McCord, 1983, Halliwell, 1987, Freeman and Crapo, 1982). The adverse health effects possibly due to oxidative damages in canine parvovirus diarrhoea can be prevented by ensuring adequate antioxidant defense.
The body’s ability to counteract oxidative stress depends on the status and activities of antioxidant molecules including the integrity of several enzymes that require adequate supply of trace minerals like zinc, copper, cobalt, selenium and manganese (Evans and Halliwell, 2001). Zinc and copper are the co-factors of many antioxidant enzymes, and are utilized for synthesis of antioxidant enzyme Cu–Zn Superoxide dismutase which catalyses conversion of superoxide radicals to less oxidizing H2O2 (Halliwell, 1994). Previous studies have shown that humans infected with HIV have deficiencies of trace minerals like selenium, and vitamin D (Cirelli et al., 1991, Simba et al., 1993) and inclusion of antioxidant with antiviral agent have been reported to have beneficial effects (Palamara et al, 1996). It was therefore hypothesized that gastroenteritis with involvement of canine parvo-virus may influence oxidative indices and that these may be targets for supportive therapy.
2. Materials and methods
2.1. Animals
Fifty six dogs with clinical signs of diarrhea and/or vomition and 14 apparently healthy dogs of identical age (1–12 months) group were recruited for the study with the consent of the owner. Dogs were randomly picked up form the canine cases presented to the Referral Veterinary Polyclinic of Indian Veterinary Research Institute, Izatnagar. The healthy dogs recruited for the study were presented to the Polyclinic either for routine health check up or vaccination, and were used to serve as controls. The study was carried out with the consent of the dog owners. The sampling from clinical cases was done for the investigation work for disease diagnosis and for conducting this experiment. The animals after examination were treated by expert clinician in the Referral Polyclinic. Patient history was collected for each of the dogs recruited for the study including the feeding and management practices. The recruited dogs were maintained mainly on home-made diet.
2.2. Clinical examination and grouping of dogs
The dogs (n = 70) recruited for the study were examined clinically as a routine practice for management of the health problem. Score was assigned to each of the three clinical signs, dehydration, depression and fecal consistency, in an individual animal based on their severity following the protocol of Walker et al. (1998) with slight modification (Table 1 ). The total clinical score was computed for each of the 70 dogs and was used to classify the dogs as healthy (n = 14; group I), mild (n = 0; group II), moderately affected (n = 9; group III) or severe (n = 47; group IV) (Table 2 ). None of the dogs had clinical score ranging between 1 and 3 and, hence none of the dogs could be placed in group II (Table 2).
Table 1.
Score assigned to each of the three different clinical sings⁎
| Scores | Faecal consistency | Depression | Dehydration |
|---|---|---|---|
| 0 | Normal faeces and well formed | Normal | Normal eyes and bright |
| 1 | Pasty faeces | Mild depression | Mild dehydration |
| Slight loss of skin elasticity | |||
| Skin tent less than 3 s | |||
| 2 | Semi-liquid faeces | Moderate depression | Moderately dehydrated |
| Skin tents greater than 3 s but less than 10 s | |||
| 3 | Watery faeces | Severe depression | Unable to stand |
| Severe dehydration | |||
| Skin tents greater than 10 s |
Walker et al. (1998) with little modification.
Table 2.
Grouping of dogs based on total clinical score
| Group | Total clinical score | Severity of the case | Number of dogs in each group |
|---|---|---|---|
| Group I | 0 | Healthy (controls) | 14 |
| Group II | 1–3 | Mild | Nil |
| Group III | 4–6 | Moderate | 9 |
| Group IV | 7–9 | Severe | 47 |
| Total | 70 |
The total clinical score was derived considering the clinical signs of fecal consistency, depression and dehydration status. Each of the three parameters was assigned a score as per the guidelines given in Table 1. None of the 70 dogs scored 1–3, so there was no animal in group II.
2.3. Blood sampling and processing
In association with routine clinical sampling, the blood samples (approximately, 2 ml) were collected from each dog before the start of any treatment by venepuncture of either cephalic or recurrent tarsal vein, and using heparin (10 IU/ml of blood) as anticoagulant. Approximately, 1 ml of blood was kept for analysis of blood micronutrients and rest of the sample was processed for separation of RBC and plasma. Blood samples were centrifuged at 2000 rpm for 5 min in a refrigerated centrifuge to separate plasma. The plasma was collected in to a clean eppendorf tube. The packed RBC was re-suspended in PBS and was centrifuged for 5 min at 5000 rpm and the supernatant was discarded. The process was repeated for three times. Finally, 1:20 dilution of RBC hemolysate was prepared in distilled water for estimation of lipid peroxide (LPO), superoxide dismutase (SOD), and catalase (CAT).
2.4. Measurement of oxidative stress indices
Erythrocytes lipid peroxides (LPO) level was determined according to the method of Placer et al. (1966). The nmol of malonaldehyde (MDA) per ml of RBC hemolysate was calculated using 1.56 × 105/mol/cm as extinction coefficient (Utley et al., 1967). Superoxide dismutase (SOD) activity was estimated as per the method of Marklund and Marklund (1974) with certain modification as suggested by Minami and Yoshikawa (1979). Catalase activity was estimated in RBC hemolysate as per the method of Cohen et al. (1970). The haemoglobin in the RBC haemolysate was estimated spectro-photometrically by the cyanomethaemoglobin method (Vankampen and Ziglstra, 1961) and the MDA level and activity of antioxidant enzymes were expressed as mg−1 of haemoglobin.
2.5. Electrolytes and micro-minerals
Plasma potassium and sodium was estimated using a flame photometer (Mediflame Electronic Corporation of India Ltd.). Blood samples were wet digested as per the method described by Kolmer et al., (1951). The concentration of copper, cobalt, zinc and iron in the digested sample was measured by atomic absorption spectrophotometer (AAS 4141, ECIL). The trace mineral concentration in blood was calculated by multiplying the corresponding dilution factor and the values were expressed in μg/g of blood.
2.6. Collection and processing of fecal samples
Fecal swabs were collected from each dog in sterile vials containing Dulbecco’s Modified Eagles Media for diagnosis of canine parvovirus infection. Genomic DNA was extracted from the fecal samples by phenol and chloroform method. Specific primer pair as reported by Senda et al. (1995) was used with slight modification to identify CPV2a/2b variants. These primers amplified a portion of VP1/VP2 gene of both CPV2a and CPV2b variants to yield a product size of 681 bp. Another pair of primers was used to amplify a 681 bp region of CPV-2 variant only (Pereira et al., 2000).
2.7. Statistical analysis
Besides classification of the 70 dogs based on the total clinical score in an individual dog, the clinically affected dogs (n = 56) were classified into two groups based on variables such as sex of the dogs, and amplification of CPV-DNA in the faecal samples. Data were analyzed to find out mean, standard error, range and significance of difference (P < 0.05), if any, between mean values of different groups classified based on different variables using one way Analysis of Variance (Snedecor and Cochran, 1994).
3. Results
The affected dogs had clinical signs of anorexia, diarrhoea, vomition and depression as observed on detailed clinical examination. Cross questioning to the owner revealed 52 out of 56 dogs (92.86%) non-vaccinated against canine distemper, hepatitis, leptospira, parvovirus and parainfluenza (DHLPPi). According to owners’ complaint, most of the dogs were suffering from diarrhoea for the last one to two days and the dogs were not taking any food. Out of these 56 cases with diarrhoea, 46 (82.14%) dogs had blood in their stool.
3.1. Identification of canine parvovirus by PCR
A total of 33 (59%) cases out of 56 affected dogs examined were found positive for parvoviral infection on faecal sample examination by PCR. Three dogs were positive for CPV-2 as evident by anticipated 681 bp product by PCR assay using the CPV-2 specific primer set. The rest of the samples were positive with the CPV-2a/2b (n = 30) specific primer set and yielded the similar 681 bp products.
3.2. Oxidative stress indices in dogs with diarrhoea
Fig. 1 shows the lipid peroxide level and antioxidant enzyme activity in erythrocytes from dogs with varying degrees of clinical severity. Significant (P < 0.05) increase in erythrocytic lipid peroxide (LPO) was recorded in both the moderately and severely affected dogs as compared to age-matched healthy controls. The activities of catalase were also significantly (P < 0.05) higher in both the clinically affected groups and that of superoxide dismutase (SOD) in moderately affected group. The activity of these two antioxidant enzymes in the erythrocytes from moderately affected dogs was significantly higher than the respective value in severely affected dogs (Fig. 1).
Fig. 1.
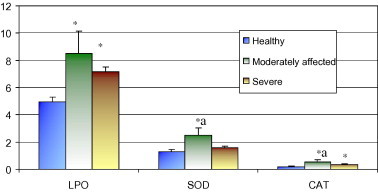
Lipid peroxide level and antioxidant enzymes activity in erythrocytes from dogs suffering from diarrhoea of varying clinical severity. LPO-lipid peroxides (nmol MDA/mg of Hb), SOD-Superoxide dismutase (Units/mg Hb), CAT-Catalase (Units/mg Hb). ∗Mean value (±SE) differing significantly at P < 0.05 from control group. aMean value differing significantly (P < 0.05) between the two affected groups.
3.3. Blood micro-minerals and plasma electrolytes
Table 3 shows the blood micro-minerals and plasma electrolytes in three different groups of dogs classified based on total clinical score. Plasma sodium remained comparable (P > 0.05) among the affected and healthy groups. Plasma potassium level was lower in both the affected groups and it reduced significantly (P < 0.05) from the control level in severely affected dogs. The mean blood copper and iron remained significantly higher in moderately affected dogs as compared to the respective levels in healthy controls; however the level in severely affected dogs remained comparable to healthy dogs. Blood zinc level reduced in affected dogs and the mean level in severely affected group was significantly lower as compared to healthy counter parts.
Table 3.
Clinical score, plasma electrolyte and blood micro-mineral status in dogs suffering from diarrhoea of varying clinical severity
| Healthy control |
Group III |
Group IV |
|
|---|---|---|---|
| (N = 14) | (N = 9) | (N = 47) | |
| Mean clinical score | 0.0 ± 0.0 | 5.1 ± 0.3 | 8.0 ± 0.14 |
| (Healthy) | (Moderately severe) | (Severe) | |
| Plasma sodium (mEq/l) | 146.7 ± 3.0 | 143.6 ± 2.0 | 141.2 ± 1.1 |
| (131.6–177.7) | (133.8–153.5) | (117.3–153) | |
| Plasma potassium (mEq/l) | 4.9 ± 0.16a | 4.5 ± 0.2ab | 4.2 ± 0.08b |
| (4.0–6.0) | (3.7–5.3) | (2.7–5.4) | |
| Blood copper (μg/g) | 0.48 ± 0.04a | 0.77 ± 0.18b | 0.60 ± 0.03ab |
| (0.29–0.74) | (0.39–2.12) | (0.19–0.95) | |
| Blood cobalt (μg/g) | 0.35 ± 0.04ab | 0.43 ± 0.09b | 0.26 ± 0.02a |
| (0.0–0.62) | (0.22–1.0) | (0.0–0.74) | |
| Blood iron (μg/g) | 116.4 ± 6.7a | 151.0 ± 32.9b | (105.6 ± 4.9a) |
| (84.0–160.1) | (69.5 ± 391.6) | (57.1–214.8 | |
| Blood zinc (μg/g) | 4.76 ± 0.17a | 3.62 ± 0.44a | 3.38 ± 0.18b |
| (3.69–5.93) | (2.62–5.6) | (0.07–6.52) |
Values are expressed as Mean ± SE values in the parenthesis denote range. Mean values with different superscripts in a row differ significantly at P < 0.05.
3.4. Grouping of dogs based on association of parvovirus in diarrhoea
The mean clinical score (8.1 ± 0.2) was higher in parvoviral gastroenteritis (n = 33) than clinical cases without involvement of parvovirus (6.60 ± 0.3, n = 23). However, the mean clinical score in CPV2 (9.00 ± 0.00, n = 3) and CPV2a/2b (8.00 ± 0.21, n = 30) were statistically (P > 0.05) comparable but the either of score was significantly (P < 0.05) higher than dogs without parvoviral infection (6.60 ± 0.3, n = 23) and that of health dogs (0.00, n = 14). Lipid peroxide level in erythrocytes from dogs suffering from parvoviral gastroenteritis was significantly (P < 0.05) higher than that of healthy controls, and of dogs without parvovirus involvement (Fig. 2 ). Erythrocytic catalase activity in dogs of either of the two affected groups remained significantly higher than that of healthy dogs, and was comparable between the two affected groups. SOD activities in the erythrocytes remained comparable between groups. There was no statistical difference between mean values of LPO (6.3 ± 0.70 and 8.5 ± 0.63 nmol of MDA/mg of Hb), SOD (1.1 ± 0.11 and 1.8 ± 0.23 U/mg of Hb) and Catalase (0.26 ± 0.02 and 0.39 ± 0.04) in dogs with CPV 2 and CPV2a/2b infection.
Fig. 2.
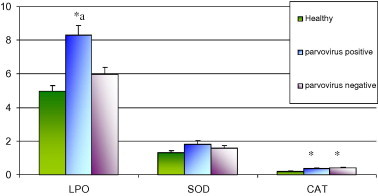
Erythrocytic oxidative stress indices in dogs suffering from diarrhoea with and without parvovirus association. 33 out of 56 dogs with diarrhoea were confirmed positive for parvovirus infection by polymerase chain reaction (PCR) of DNA extracted from faecal samples. LPO-lipid peroxides (nmol MDA/mg of Hb), SOD-Superoxide dismutase (Units/mg Hb), CAT-Catalase (Units/mg Hb). ∗Mean value (±SE) differing significantly at P < 0.05 from control group, and aMean value differing significantly (P < 0.05) from that of cases with gastroenteritis without parvoviral infection.
Plasma sodium and potassium levels were significantly (P < 0.05) lower in dogs without parvoviral infection as compared to that of healthy controls, and the levels were statistically comparable between the two affected groups irrespective of parvovirus involvement (Table 4 ). The blood zinc concentration in healthy dogs remained significantly (P < 0.05) higher than that of either of the affected groups and it was lower in dogs with parvoviral involvement. There was no statistical difference between the two affected groups with respect to blood copper, cobalt and iron concentrations.
Table 4.
Clinical score, plasma electrolytes profile and blood micro-minerals in different groups of dogs grouped based on parvoviral involvement in gastroenteritis
| Healthy control | Gastroenteritis associated with parvovirus infection | Gastroenteritis without parvoviral etiology | |
|---|---|---|---|
| (n = 14) | (n = 33) | (n = 23) | |
| Clinical score | 0.0 ± 0.0a | 6.60 ± 0.3b | 8.1 ± 0.2c |
| (4.0–9.0) | (5.0–9.0) | ||
| Plasma sodium (mEq/l) | 146.7 ± 2.95b | 142.5 ± 1.1ab | 140.1 ± 1.7a |
| (131.6–177.7) | (129.4–159) | (117.3–153.5) | |
| Plasma potassium (mEq/l) | 4.92 ± 0.16b | 4.23 ± 0.1a | 4.30 ± 0.13a |
| (4.0–6.0) | (2.9–5.4) | (2.7–5.3) | |
| Copper (μg/g) | 0.48 ± 0.04 | 0.64 ± 0.06 | 0.60 ± 0.04 |
| (0.29–0.74) | (0.19–2.12) | 0.16–1.35 | |
| Cobalt (μg/g) | 0.34 ± 0.04 | 0.28 ± 0.03 | 0.29 ± 0.04 |
| 0.00–0.62 | (0.10–1.05) | 0.00–0.74 | |
| Iron (μg/g) | 116.4 ± 6.6 | 113.0 ± 10.7 | 112.7 ± 7.0 |
| (84–160) | (65.3–391.6) | (57.0–188.5) | |
| Zinc (μg/g) | 4.76 ± 0.17c | 3.36 ± 0.23a | 3.5 ± 0.2b |
| (3.69–5.93) | (0.07–6.52) | (2.03–6.22) |
Values are expressed as Mean ± SE. Values in the parenthesis denotes range. Mean values with dissimilar superscripts in a row differ significantly at P < 0.05. Out of 55 clinical cases of diarrhoea, 33 were confirmed to have parvoviral infection by facial sample examination by PCR.
3.5. Oxidative stress indices and blood micro minerals profile with respect to sex variable
The mean erythrocytic LPO level and activity of SOD and catalase in male (n = 35) and female (n = 21) dogs with clinical signs of diarrhoea (n = 56) irrespective of parvovirus confirmation in faecal samples, total clinical score, breed or age of the dogs, remained statistically (P > 0.05) comparable (Table 5 ) indicating that either sex of dogs is equally susceptible to oxidative stress in gastroenteritis. Table 6 shows the plasma sodium and potassium and blood copper, cobalt, zinc and iron concentrations in dogs of both the sexes with signs of diarrhoea. All the parameters remained comparable (P > 0.05) between two sexes of dogs with signs of diarrhoea except copper that remained significantly higher in female dogs than males.
Table 5.
Influence of Sex of the dogs on erythrocytic oxidative stress indices with clinical signs of diarrhoea
| Number of dogs | Female |
Male |
Total |
|---|---|---|---|
| 21 | 35 | 56 | |
| LPO (nmol MDA/mg of Hb) | 7.1 ± 0.7 | 7.5 ± 0.5 | 7.36 ± 0.41 |
| (3.59–17.8) | (3.0–15.9) | (3.0–17.7) | |
| SOD (units/mg Hb) | 1.6 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.14 |
| (0.6–4.4) | (0.6–5.3) | (0.55–5.27) | |
| Catalase (units/ mg Hb) | 0.37 ± 0.05 | 0.39 ± 0.04 | 0.39 ± 0.03 |
| (0.16–0.93) | (0.19–1.35) | (0.16–1.35) |
Values are expressed as Mean ± SE. Values in the parenthesis denote range.
Table 6.
Effect of the sex on blood micro-minerals and plasma electrolytes profile in dogs with clinical signs of diarrhoea
| Number of dogs | Female |
Male |
Total |
|---|---|---|---|
| 21 | 35 | 56 | |
| Plasma sodium (mEq/l) | 140.0 ± 1.7 | 142.5 ± 1.14 | 141.55 ± 0.97 |
| Plasma potassium(mEq/l) | 4.0±0.13 | 4.36 ± 0.09 | 4.3 ± 0.08 |
| Blood copper (μg/g) | 0.73 ± 0.08⁎ | 0.56 ± 0.03 | 0.63 ± 0.04 |
| Blood cobalt (μg/g) | 0.29 ± 0.05 | 0.28 ± 0.02 | 0.29 ± 0.02 |
| Blood iron (μg/g) | 127.3 ± 15.2 | 104.3 ± 5.8 | 112.9 ± 6.9 |
| Blood zinc (μg/g) | 3.35 ± 0.34 | 3.45 ± 0.16 | 3.41 ± 0.16 |
Values are expressed as Mean ± SE.
Differ significantly at P < 0.05 between the sex.
4. Discussion
This is the first report documenting the involvement of oxidative stress in gastroenteritis in dogs with parvoviral infection. Diarrhoea and vomition are the common problems encountered in canine practice and are the most common manifestations of gastroenteritis, an inflammatory condition of stomach and intestine. Viral etiologies like canine parvo and corona viruses are the most common agents among different causes of gastroenteritis. In the present study, the majority of the dogs (52 out of 56) were unvaccinated and such vaccination trends are not uncommon (Houston et al., 1996). The parvoviral involvement was recorded in 59% of cases with the signs of diarrhoea/ vomition and such cases had a higher clinical score as compared to diarrhoea without parvovirus being diagnosed in faecal samples. In the present investigation, dogs aged between one and 12 months were recruited for the study as the incidence of parvoviral gastroenteritis has been reported to be the highest in puppies between weaning and 6 month of age (Houston et al., 1996).
The reactive oxygen and nitrogen species play a complex role in many diseases and in metabolic regulation in a disease process, and oxidative stress has been implicated in several viral infections in man and animals. Virus-induced oxidative stress could be mediated by an early phase of liberation of pro-inflammatory cytokines. Participation of iron in Fenton reaction in vivo leads to production of more reactive hydroxyl radicals from superoxide radicals and H2O2 (Halliwell, 1994) and results in increased lipid peroxidation. This might be one of the reasons for significant alteration in LPO in dogs suffering from gastroenteritis irrespective of parvoviral involvement in the present study.
Antioxidant enzyme levels are sensitive markers of oxidative stress. Both increased and decreased levels have been reported in different diseases as a consequence of enhanced ROS production either by up-regulation of enzyme activity or utilization of the antioxidant enzymes to counter the ROS. In the present investigation, a state of up-regulation of synthesis and activity of antioxidant enzymes in moderately affected dogs and a state of un-compensatory oxidative damage in severely affected dogs was observed. Altered status of antioxidant enzymes was documented in viral diseases like dengue haemorrhagic fever (Gil et al., 2004) and viral influenza (Oda et al., 1989). Increased SOD and catalase activity in the present investigation might be due to enhanced synthesis of antioxidant enzymes in moderately severe cases of gastroenteritis as in-built compensatory mechanism. Increased oxidative stress indices in dogs have been reported in other infections such as Hepatozoon canis (Kiral et al., 2005), Lieshmania (Bildik et al., 2004) and in undifferentiated diarrhea in other animal species (Ranjan et al., 2006). There appears to have no report on oxidative stress indices in dogs due to viral infections to compare with the present findings.
Oxidative stress indices did not show statistical (P > 0.05) variation when the dogs are grouped as male or female, irrespective of etiology, breed, age, or clinical score of gastroenteritis. However, Ide et al. (2002) reported that oxidative stress is greater in males than in females due to greater generation of ROS and reduced antioxidant activity. Houston et al. (1996) reported that male dogs >6 month of age are at higher risk of developing parvoviral enteritis but both male and female young dogs <6 month of are equally susceptible and this might be the reason that there was no difference in oxidative stress indices in male and female pups.
Trace elements are integral part of cellular antioxidant system and elements like copper, zinc and selenium participate in cellular defense against oxidants (Klotz et al., 2003). Cells of the immune system require an adequate supply of trace elements for the structure and function of metalloproteins that participate in normal maintenance processes like protection of the cells against highly toxic reactive oxygen species (e.g. copper for superoxide dismutase and iron for catalase) (Munoz et al., 2007). Significantly increased level of blood copper was observed in dogs suffering from gastroenteritis irrespective of presence of canine parvovirus. Increased level of copper in the blood of affected animals might be due to increased inflammatory response as copper is an integral part of protein ceruloplasmin and its level rises in acute phase response. A decreased level of blood iron and copper in the severely affected group might be due to loss of blood as many dogs in the severely affected group had blood in their stool.
Zinc is a component of Cu–Zn Superoxide dismutase (Bray and Bettger, 1990) that converts the superoxide radicals to hydrogen peroxide, which is further decomposed by catalase into water and oxygen. Significantly low level of blood zinc and increase in blood copper level were observed in dog suffering from diarrhea irrespective of presence of canine parvovirus. There are reports indicating significant effect of oral zinc supplementation on morbidity in acute diarrhea in children (Sazawal et al., 1997, Wingertzahn et al., 2003). In past few years there are several reports supporting the hypothesis that host micronutrient status may contribute towards disease pathogenesis and emergence of new virulent strains of viruses (Beck et al., 2004) and nutritionally compromised hosts are more susceptible to viral infection (Beck and Levander, 1998). Khoo et al, (2005) showed that animals on high antioxidant foods had significantly increased titers and memory cells during canine parvovirus and canine distemper virus vaccination.
It is concluded that clinical problem gastroenteritis in dogs was associated with increased levels of lipid peroxides and alteration in antioxidant enzymes. The alterations were more pronounced with the involvement of canine parvovirus (CPV) with non-significant changes between CPV and CPV2a/2b, indicating a state of oxidative stress and suggesting that incorporation of antioxidants in therapeutic regimen or enhancing the redox status of patient during gastroenteritis, more particularly in canine parvoviral diarrhoea may help in ameliorating the disease process.
Acknowledgement
Financial assistance in the form of Indian Council of Agricultural Research (New Delhi)-Junior Research Fellowship to the first author is gratefully acknowledged.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.rvsc.2008.05.008.
Appendix A. Supplementary data
References
- Allard J.P., Aghdassi E., Chau J., Salit I., Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. American Journal of Clinical Nutrition. 1998;67:143–147. doi: 10.1093/ajcn/67.1.143. [DOI] [PubMed] [Google Scholar]
- Beck M.A., Levander O.A. Dietary oxidative stress and the potentiation of viral infection. Annual Review Nutrition. 1998;18:93–116. doi: 10.1146/annurev.nutr.18.1.93. [DOI] [PubMed] [Google Scholar]
- Beck M.A., Handy J., levander O.A. Host nutritional status: the neglected virulence factor. Trends in Microbiology. 2004;12:417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bildik A., Kargin F., Seyrek K., Pasa S., Ozensoy S. Oxidative stress and non-enzymatic antioxidative status in dogs with visceral Leishmaniasis. Research in Veterinary Science. 2004;77:63–66. doi: 10.1016/j.rvsc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Bray T.M., Bettger W.J. The physiological role of zinc as an antioxidant. Free Radical Biology and Medicine. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- Carmichael L.E. An annotated historical account of canine parvovirus. Journal of Veterinary Medicine B. 2005;52:303–311. doi: 10.1111/j.1439-0450.2005.00868.x. [DOI] [PubMed] [Google Scholar]
- Cirelli A., Ciardi M., de Simone C., Sorice F., Giordano R., Ciaralli L., Constatini S. Serum selenium concentration and disease progress in patient with HIV infection. Clinical Biochemistry. 1991;24:211–224. doi: 10.1016/0009-9120(91)90601-a. [DOI] [PubMed] [Google Scholar]
- Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Analytical Biochemistry. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- de Mari K., Maynard L., Eun H.M., Lebreux B. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled field trial. Veterinary Record. 2003;152:105–108. doi: 10.1136/vr.152.4.105. [DOI] [PubMed] [Google Scholar]
- Evans P., Halliwell B. Micronutrients: oxidant and antioxidant status. British Journal of Nutrition. 2001;85(Suppl. 2):S67–S74. [PubMed] [Google Scholar]
- Freeman B.A., Crapo J.D. Biology of disease. Free radicals and tissue injury. Laboratory Investigation. 1982;47:412–426. [PubMed] [Google Scholar]
- Gil L., Martinez G., Tapanes R., Castro O., Gonzalez D., Bernardo L., Vazquez S., Kouri G., Guzman M.G. Oxidative stress in adult dengue patients. American Journal of Tropical Medicine and Hygiene. 2004;71:652–657. [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concept. FASEB Journal. 1987;1:358–364. [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause and consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Houston D.M., Ribble C.S., Head L.L. Risk factors associated with parvovirus enteritis in dogs: 283 cases (1982–1991) Journal of American Veterinary Medical Association. 1996;208:542–546. [PubMed] [Google Scholar]
- Ide T., Tsutsu H., Ohashi N., Hayashidani S., Suematsu N., Tsuchihashi M., Tamai H., Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- Khoo C., Cunnick J., Friesen K., Gross K.L., Wedekind K., Jewell D.E. The role of supplementary dietary antioxidants on immune response in puppies. Veterinary Therapeutics. 2005;6:43–56. [PubMed] [Google Scholar]
- Kiral F., Karagenc T., Pasa S., Yenisey C., Seyrek K. Dogs with hepatozoon canis respond to the oxidative stress by increased production of glutathione and nitric oxide. Veterinary Parasitology. 2005;131:15–21. doi: 10.1016/j.vetpar.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Klotz L.O., Kroncke K.D., Buchczyk D.P., Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. Journal of Nutrition. 2003;133 5(Suppl 1):1448S–1451S. doi: 10.1093/jn/133.5.1448S. [DOI] [PubMed] [Google Scholar]
- Kolmer J.A., Spaulding E.H., Robinson H.W. Appletion Century Crafts; New York: 1951. Approved Laboratory Techniques. pp. 190–191. [Google Scholar]
- Kumar A., Varshney J.P., Patra R.C. A comparative study on oxidative stress in dogs infected with Ehrlichia canis with or without concurrent infection with Babesia gibsoni. Veterinary Research Communications. 2006;30:917–920. doi: 10.1007/s11259-006-3365-6. [DOI] [PubMed] [Google Scholar]
- Martin V., Najbar W., Gueguen S., Grousson D., Eun H.M., Lebreux B., Aubert A. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled challenge trial. Veterinary Microbiology. 2002;89:115–127. doi: 10.1016/s0378-1135(02)00173-6. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McCord J.M. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983;94:412–414. [PubMed] [Google Scholar]
- Minami M., Yoshikawa H. Simplified assay method of superoxide dismutase activity of clinical use. Clinca Chimica Acta. 1979;92:337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Munoz C., Rios E., Olivos J., Brunser O., Olivares M. Iron, copper and immunocompetence. British Journal of Nutrition. 2007;98(Suppl. 1):S24–S28. doi: 10.1017/S0007114507833046. [DOI] [PubMed] [Google Scholar]
- Murase T., Ueda T., Yamato O., Tajima M., Maede Y. Oxidative damage and enhanced erythrophagocytosis in canine erythrocytes infected with Babesia gibsoni. Journal of Veterinary Medical Science. 1996;58:259–261. doi: 10.1292/jvms.58.259. [DOI] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989;244:974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Palamara A.T., Garaci E., Rotilio G., Ciriolo M.R., Casabianca A., Fraternale A., Rossi L., Schiavano G.F., Chiarantini L., Magnani M. Inhibition of murine AIDS by reduced glutathione. AIDS Research and Human Retroviruses. 1996;12:1373–1381. doi: 10.1089/aid.1996.12.1373. [DOI] [PubMed] [Google Scholar]
- Pereira C.A.D., Monezei T.A., Mehnert D.U., Angelo M.D., Durigon E.L. Molecular characterization of canine parvovirus in Brazil by polymerase chain reaction assay. Veterinary Microbiology. 2000;75:127–132. doi: 10.1016/s0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- Peterhans E., Grob M., Burge T., Zanoni R. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radical Research Communications. 1987;3:39–46. doi: 10.3109/10715768709069768. [DOI] [PubMed] [Google Scholar]
- Placer Z.A., Cushman L., Johnson B. Estimation of product of lipid peroxidation (Malonydialdehyde) in biochemical system. Analytical Biochemistry. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Ranjan R., Naresh R., Patra R.C., Swarup D. Erythrocyte lipid peroxides and blood zinc and copper concentration in acute undifferentiated diarrhea in calves. Veterinary Research Communications. 2006;30:249–254. doi: 10.1007/s11259-006-3185-8. [DOI] [PubMed] [Google Scholar]
- Sazawal S., Black R.E., Bhan M.K., Jalla S., Sinha A., Bhandari N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea – a community-based, double-blind, controlled trial. American Journal of Clinical Nutrition. 1997;66:413–418. doi: 10.1093/ajcn/66.2.413. [DOI] [PubMed] [Google Scholar]
- Semba R.D., Tang A.M. Micronutrients and the pathogenesis of human immunodeficiency virus infection. British Journal of Nutrition. 1999;81:181–189. doi: 10.1017/s0007114599000379. [DOI] [PubMed] [Google Scholar]
- Senda M., Parrish C.R., Harasawa R., Gamoh K., Muramatsu M., Hirayama N., Itoh O. Detection by PCR of wild type canine parvovirus which contaminates dog vaccine. Journal of Clinical Microbiology. 1995;33:110–113. doi: 10.1128/jcm.33.1.110-113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simba R.D., Graham N.M.H., Caiffa W.T. Increased mortality associated with vitamin D deficiency during human immunodeficiency virus type 1 infection. Archives of Internal Medicine. 1993;153:2149–2154. [PubMed] [Google Scholar]
- Snedecor G.W., Cochran W.G. sixth ed. Oxford and IBH Publishing Co.; New Delhi: 1994. Statistical Methods. [Google Scholar]
- Utley H.G., Bernheim F., Hochsein P. Effect of sulphydryl reagents on peroxidation of microsomes. Archives of Biochemistry and Biophysics. 1967;118:29–32. [Google Scholar]
- Vankampen E.J., Ziglstra W.G. Colorimetric determination of haemoglobin. Clinical Chemistry Acta. 1961;6:5388. [Google Scholar]
- Wingertzahn M.A., Rehman K.U., Altaf W., Wapnir R.A. Zinc as a potential enteroprotector in oral rehydration solutions: its role in nitric oxide metabolism. Paediatric Research. 2003;53:434–439. doi: 10.1203/01.PDR.0000049465.73687.4D. [DOI] [PubMed] [Google Scholar]
- Walker P.G., Constable P.D., Morrin D.E., Foreman J.H., Drakley J.K., Thurman J.C. Comparison of hypertonic saline dextran solution and lactated Ringer’s solution for resuscitating severely dehydrated calves with diarrhoea. Journal of American Veterinary Medical Association. 1998;213:113–121. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


