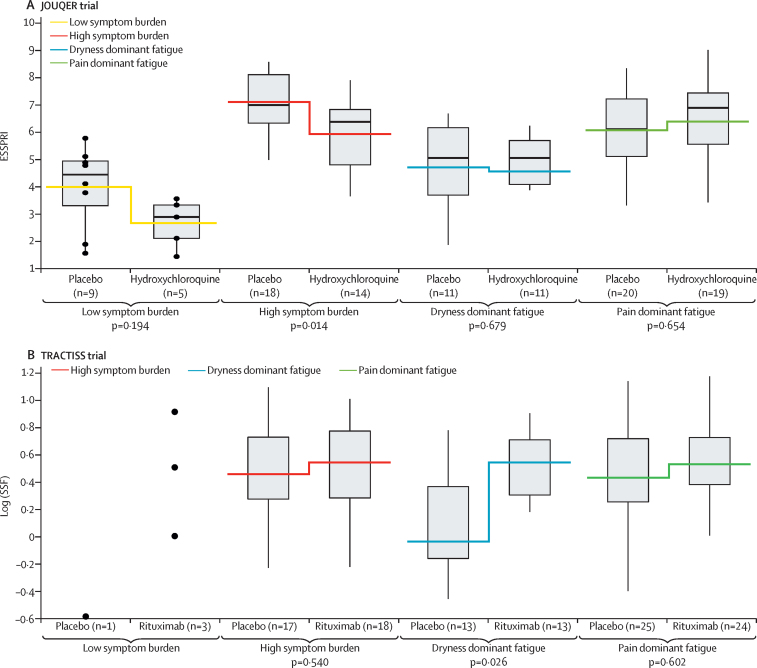
Figure 3.
Reanalysis of two clinical trials using symptom-based subgroups
(A) ESSPRI scores for each subgroup for patients in placebo and HCQ groups in the JOQUER trial.17 Box plots show the median ESSPRI scores, quartiles, and ranges for placebo and hydroxychloroquine for LSB, HSB, DDF, and PDF subgroups. The step break indicates the mean ESSPRI scores of the placebo and hydroxychloroquine treatments for each subgroup. Although we found no overall treatment effect, we found a significant treatment by subgroup interaction. This consistency test is statistically significant (p=0·036). The p values shown are for the contrast within each subgroup. (B) Stimulated salivary flow for each subgroup for patients in the placebo and rituximab groups of the TRACTISS trial.18 Box plots of log transformed data show the median SSF and ranges for placebo and rituximab treatments for each subgroup. Data are shown for the LSB subgroup; however, statistical analysis was not done because of insufficient data in this stratum. Although the figures show group values at the end of the trial, the probability values refer to the statistical analysis on changes from baseline as per the original clinical protocols. LSB=low symptom burden. HSB=high symptom burden. DDF=dryness dominant with fatigue. PDF=pain dominant with fatigue. SSF=stimulated salivary flow.