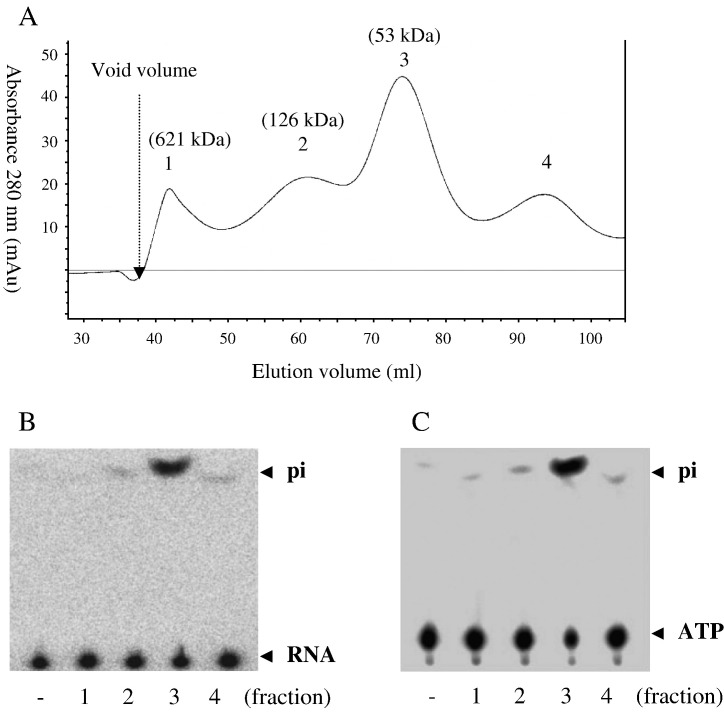
Fig. 1.
Oligomerization states and activities of the BaMV helicase-like domain. (A) Gel filtration elution profile. The molecular mass of the refolded protein in each collected fraction (peaks 1–3) was estimated as described in Materials and methods. (B) RTPase. The protein in each collected fraction (30 ng each) was incubated with [γ-32p]RNA (0.4 μg) in a final 3 μl solution at 20 °C for 1 min. “−” indicates a control without addition of enzyme. The reaction products were resolved on TLC plates and visualized by autoradiography. (C) ATPase. The conditions were as in part B except that 0.2 mM ATP, containing 1 μCi [γ-32p]ATP, was used as the substrate.