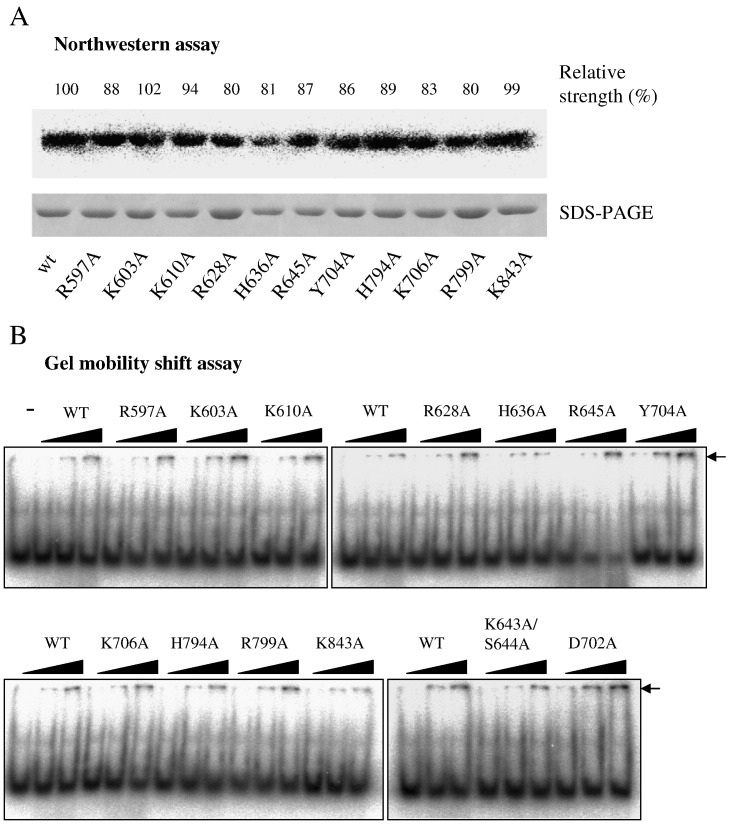
Fig. 3.
The relative RNA-binding strength of variant BaMV helicase-like domains. (A) Northwestern assay. Proteins resolved on SDS-PAGE were transferred to nitrocellulose and allowed to refold in buffer as described in Materials and methods. After incubation with 32P-labeled RNA for 60 min, the membrane was visualized with a phosphorimager. The relative ability to bind the RNA probe in comparison to WT is indicated after normalization to the loaded protein. (B) Gel mobility shift assay. Different amounts (50, 130, or 260 ng) of the indicated protein were incubated with a 50-nt 32P-labeled RNA (50 ng) at 20 °C for 1 h prior to electrophoretic separation on 8% PAGE as described in Materials and methods. “−” indicates the migration of RNA probe in the absence of protein.