Figure 6.
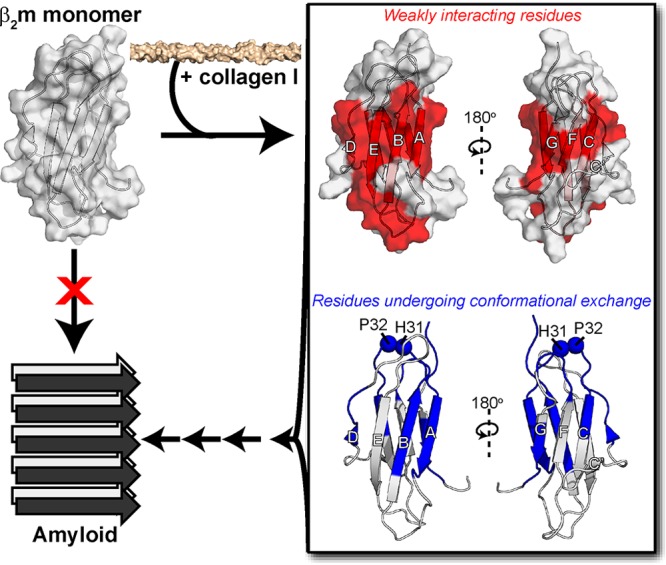
Proposed mechanism for collagen-driven β2m amyloidogenesis. Alone, the β2m monomer (PDB: 2XKS)49 does not readily aggregate into amyloid fibrils. Upon addition of collagen I, we have observed interaction interfaces to include both β-sheets of β2m through the 15N-DEST experiment (red). Collagen also induces conformational exchange in regions colored in blue, as assessed by 15N relaxation experiments. The interaction of collagen I with the structured regions of β2m enhances conformational exchange, promoting formation of amyloid-competent species and inducing aggregation.
