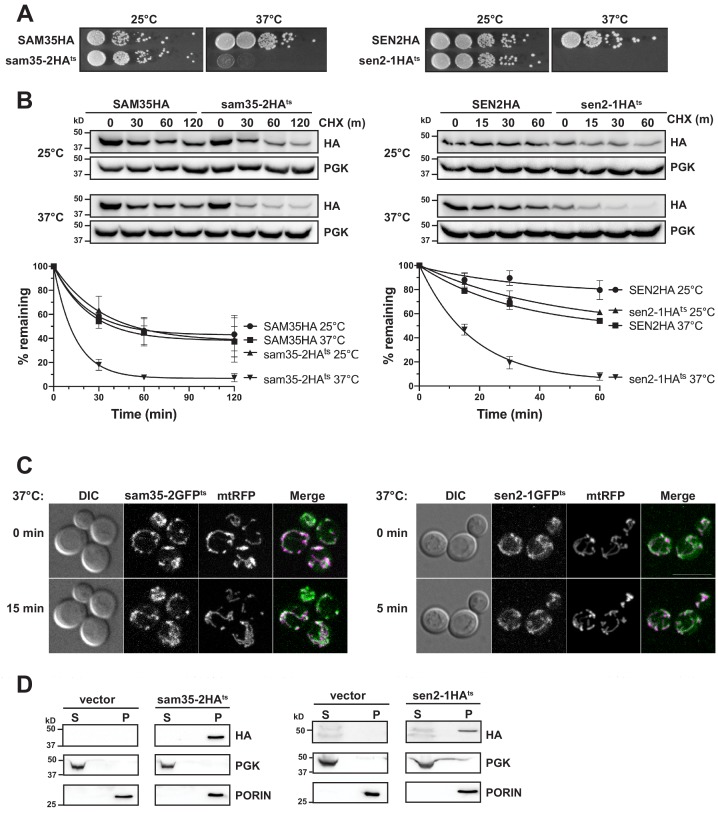
Figure 1. The temperature sensitive proteins sam35-2HAts and sen2-1HAts are novel thermosensitive substrates for mitochondrial quality control.
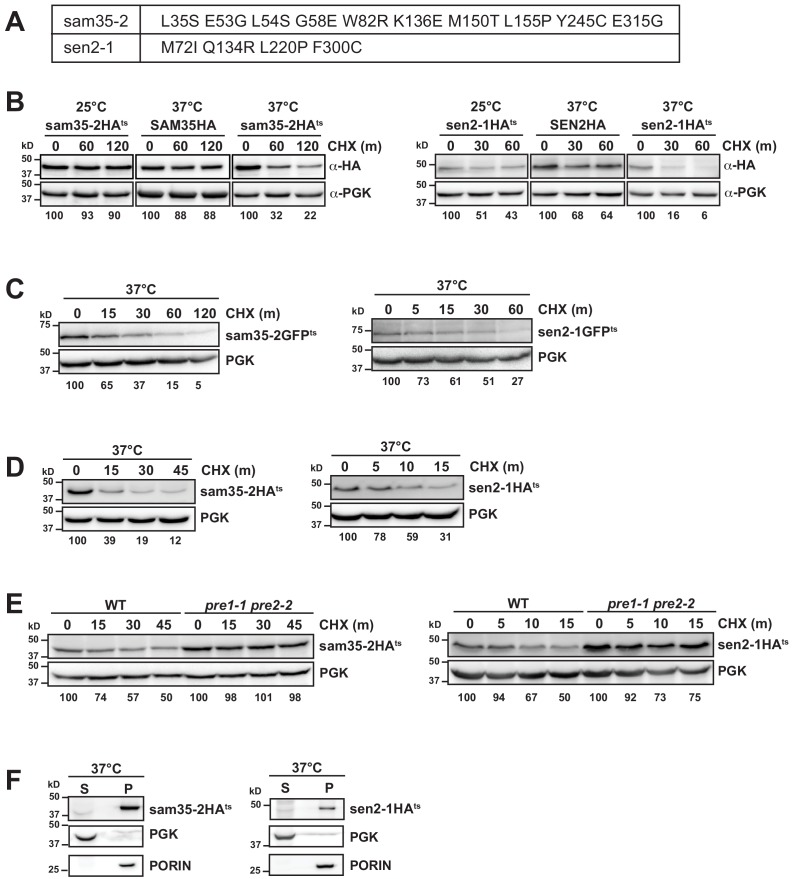
(A) Spot growth assay of cells expressing chromosomal SAM35HA, sam35-2HAts, SEN2HA, or sen2-1HAts (yMM36, 37, 40, and 41, respectively) at permissive (25°) or non-permissive (37°) temperatures. (B) Wild type (WT; WCG4a) yeast were treated with cycloheximide (CHX) at 25°C or 37°C and analyzed at the indicated times to assess the degradation of centromeric (CEN) plasmid-expressed SAM35HA, sam35-2HAts, SEN2HA, or sen2-1HAts (pMM158, 157, 159, 160, respectively). The ts- proteins were detected by immunoblotting with HA antibody. Phosphoglycerate kinase (PGK) served as a protein loading control. Graphed below is the mean and standard deviation (SD) of the PGK-normalized HA signal at each time point for three biological replicates. (C) Live-cell microscopy analysis of agarose-embedded WT cells (WCG4a) co-expressing a mitochondrial-matrix targeted RFP (mtRFP; pMD12) and either sam35-2GFPts (pMD1) or sen2-1GFPts (pMD4) at the indicated times after temperature shift to 37°C. CHX was also added at 0 min, although CHX diffusion through agarose is likely problematic. ‘Merge’ of GFP (green) and RFP (magenta) channels and differential interference contrast (DIC) are shown; Scale bar = 10 μm. (D) Lysates of spheroplasted yeast from the strains used in B were fractionated at 12,000xg at 37°C into mitochondrial pellet (P) and post-mitochondrial supernatant (S). Fractions were subject to immunoblotting with antibodies to HA, PGK (cytosolic protein control), and PORIN (mitochondrial protein control).